中国沙漠 ›› 2020, Vol. 40 ›› Issue (4): 52-62.DOI: 10.7522/j.issn.1000-694X.2020.00014
张振清1,4( ), 张昺林3, 张威1(
), 张昺林3, 张威1( ), 刘光琇1, 陈拓3, 刘阳2,5, 陈警伟6, 田茂1,4
), 刘光琇1, 陈拓3, 刘阳2,5, 陈警伟6, 田茂1,4
收稿日期:2019-11-23
修回日期:2020-01-17
出版日期:2020-08-20
发布日期:2020-09-01
通讯作者:
张威
作者简介:张威(E-mail: ziaoshen@163.com)基金资助:
Zhenqing Zhang1,4( ), Binglin Zhang3, Wei Zhang1(
), Binglin Zhang3, Wei Zhang1( ), Guangxiu Liu1, Tuo Chen3, Yang Liu2,5, Jingwei Chen6, Mao Tian1,4
), Guangxiu Liu1, Tuo Chen3, Yang Liu2,5, Jingwei Chen6, Mao Tian1,4
Received:2019-11-23
Revised:2020-01-17
Online:2020-08-20
Published:2020-09-01
Contact:
Wei Zhang
摘要:
黑戈壁区域具有干旱、强辐射等极端环境条件,鲜有人类踪迹,相关生物学研究报道较少,其中微生物研究未见报道。本研究首次针对黑戈壁生态系统中微生物分布特征开展研究,对河西走廊黑戈壁生态系统中不同生境土壤样品进行可培养细菌分离。结果表明:河西走廊黑戈壁生态系统中每克土壤可培养细菌数量(CFU)为2.3×104~1.49×106,不同生境土壤的可培养细菌具有明显差异,可培养细菌主要富集于石下生境,黑戈壁中砾石下为微生物提供了相对较适宜的生境;土壤总碳是影响黑戈壁土壤细菌数量的主要因素。结合16 S rRNA基因序列比对,分析共鉴定可培养细菌118株菌株,菌株主要归类于放线菌门(Actinobacteria)、厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)和异常球菌-栖热门(Deinococcus-Thermus)4个类群,其中放线菌门和厚壁菌门是优势门;芽孢杆菌属(Bacillus)、链霉菌属(Streptomyces)是优势属,10株细菌菌株为潜在新种。从分离菌株中筛选出了多株抗辐射活性较高的菌株,其中7株活性显著高于阳性对照耐辐射奇球菌(Deinocccus radiodurans),为进一步筛选研究细菌抗辐射机制及抗辐射活性物质提供菌株资源。
中图分类号:
张振清, 张昺林, 张威, 刘光琇, 陈拓, 刘阳, 陈警伟, 田茂. 河西走廊黑戈壁生态系统中可培养细菌分布特征及抗辐射活性[J]. 中国沙漠, 2020, 40(4): 52-62.
Zhenqing Zhang, Binglin Zhang, Wei Zhang, Guangxiu Liu, Tuo Chen, Yang Liu, Jingwei Chen, Mao Tian. Distribution characteristics and anti-radiation activity of culturable bacteria in black gobi ecosystem of the Hexi Corridor[J]. Journal of Desert Research, 2020, 40(4): 52-62.
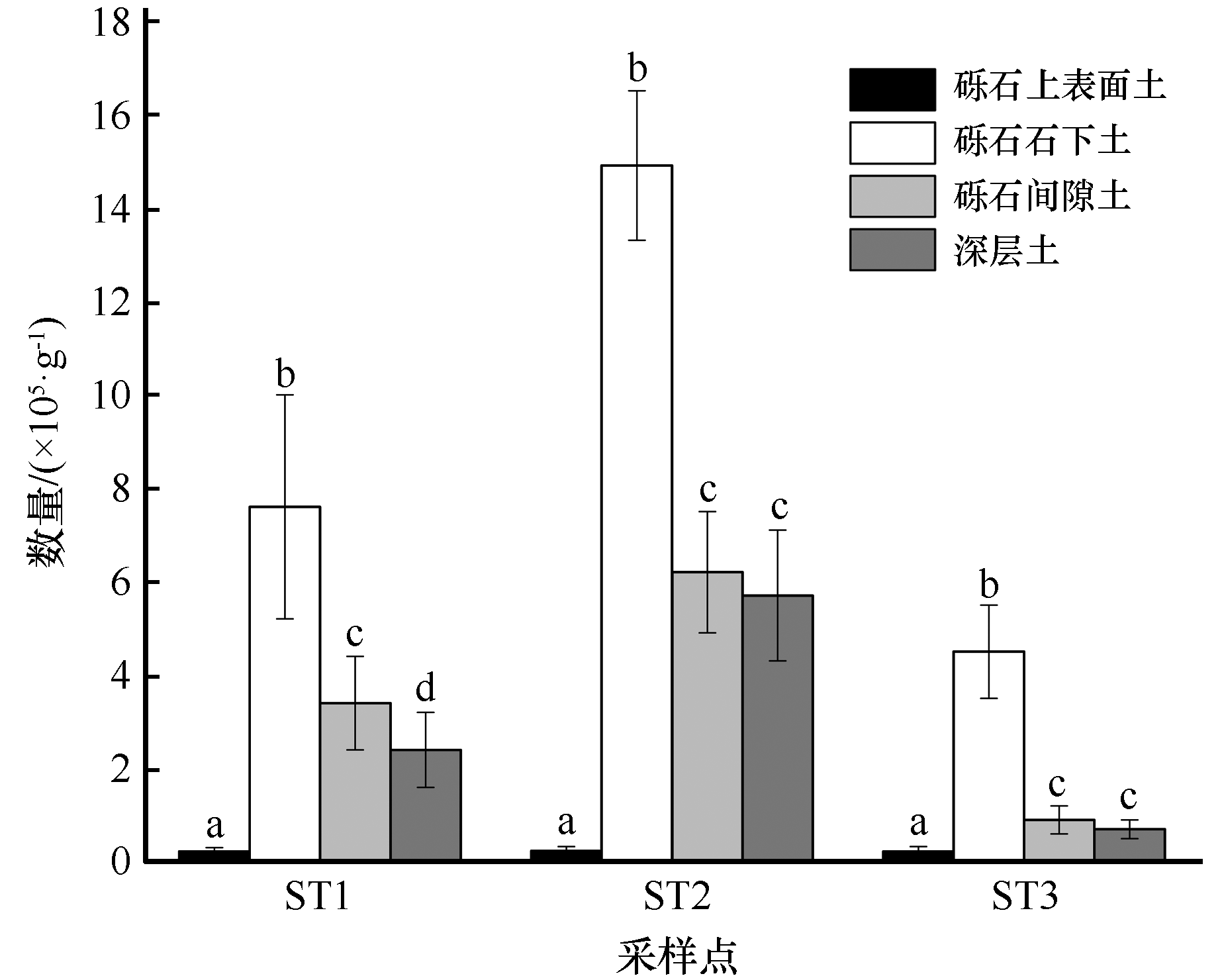
图3 河西走廊黑戈壁土壤可培养细菌数量分布特征对同一采样点,不同小写字母表示差异显著(P<0.05),相同小写字母表示差异不显著
Fig.3 Distribution characteristics of soil culturable bacteria in black gobi of the Hexi Corridor
| 门 | 属 | 编号 | 相似菌株 | 登录号 | 相似度/% | 属 | 编号 | 相似菌株 | 登录号 | 相似度/% |
|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Agrococcus | ST2LB-2 | A. citreus ZBGKL11 | KJ734880 | 99.71 | Streptomyces | ST2SX-8 | S. africanus E3SQ | MH472998 | 98.80 |
| ST1-1-2-2 | A. jenensis DW414 | KR856324 | 97.32 | ST1-1-2-5 | S. cacaoi Ru87 | KY818662 | 97.87 | |||
| ST3-6 | A. jenensis Y25 | MK721042 | 99.34 | ST3-3-2-5 | S. capoamus JCM 4734 | NR_040856 | 99.56 | |||
| ST3-4 | A. jenensis Y14 | MK721034 | 99.26 | ST3-3-2-6-1 | S. chryseus HBUM174847 | EU841613 | 97.51 | |||
| ST3-3 | A. terreus BT116 | MH934923 | 99.85 | ST2-S1 | S. coeruleoaurantiacus K7 | KR023963 | 99.85 | |||
| Amycolatopsis | ST3-18 | A. nigrescens CSC17Ta-90 | NR_043880 | 97.93 | ST1-1-1-13 | S. coeruleofuscus MR-18 | KY753217 | 99.85 | ||
| Arsenicicoccus | ST1-1-1-17-1 | A. bolidensis CCUG 47306 | NR_025598 | 99.93 | ST1SX-8 | S. glaucescens NRRL B-2706 | NR_115773 | 99.93 | ||
| Arthrobacter | ST3-16 | A. agilis IHBB 11164 | KR085842 | 99.01 | ST1SX-14 | S. gobitricini LMG 19910 | AJ781335 | 100.00 | ||
| ST3-15 | A. agilis II/11 | KM036066 | 99.71 | ST3-2-1-10-2 | S. lavendulocolor NBRC 12881 | NR_112317 | 99.93 | |||
| ST1-1-2-7 | A. crystallopoietes MR-15 | KY753214 | 99.93 | ST3B1 | S. litmocidini HBUM175011 | FJ486428 | 98.65 | |||
| Auraticoccus | ST3-10 | A. monumenti AL12 | KU258209 | 98.17 | ST1-1-2-4 | S. lunaelactis 244-HNR7 | MF077012 | 99.11 | ||
| ST3-2 | A. monumenti MON 2.2 | LT629688 | 99.55 | ST3-24-1-1 | S. lunaelactis MM109 | CP026304 | 99.12 | |||
| ST3-21 | A. sp. R-68201 | KY386505 | 95.24 | ST1-1-2-3 | S. lunaelactis MMun143 | MG980181 | 99.19 | |||
| ST3-22 | A. monumenti AL12 | KU258209 | 94.31 | ST3-2-1-1-2-1 | S. misionensis 12-4 | KJ571074 | 99.49 | |||
| Blastococcus | ST1-1-1-16 | B. aggregatus 1P10AnA | EU977831 | 99.63 | ST3-2-1-8-1 | S. misionensis cfcc3147 | FJ792563 | 99.63 | ||
| ST3-5-2 | B. capsensis R9 | MK696404 | 99.63 | ST1-1-2-10-1 | S. palmae CMU-AB199 | LC413945 | 98.02 | |||
| ST3-24-1-3 | B. capsensis RQ2 | MK696394 | 99.09 | ST1-1-2-10 | S. palmae CMU-AB204 | NR_152026 | 97.47 | |||
| ST2B4 | B. endophyticus YIM 68236 | NR_108608 | 99.32 | ST2SB-6 | S. piloviolofuscus 174468 | EU593715 | 97.66 | |||
| ST3-12-1 | B. saxobsidens DD2 | FO117623 | 99.78 | ST3-2-2-2 | S. pulveraceus MR-27 | KY753226 | 96.65 | |||
| ST3-24 | B. saxobsidens BC444 | NR_117019 | 99.33 | ST3-3-2-6 | S. sp. Z594b | MN371360 | 97.72 | |||
| ST2SX-1 | B. saxobsidens BC448 | NR_025482 | 99.64 | ST2SX-5-2 | S. rimosus PSK5-20B | MN421096 | 96.81 | |||
| Crossiella | ST3SX-11 | C. equi NRRL B-24104 | NR_025088 | 99.18 | ST1-3-2-2 | S. sioyaensis W24 | KP718602 | 99.12 | ||
| Janibacter | ST1-1-2-11 | J. terrae CS12 | NR_036868 | 99.85 | ST3-2-1-9-1 | S. spinoverrucosus 173372 | EU570683 | 98.89 | ||
| Kineococcus | ST2SS-1 | K. radiotolerans SRS30216 | NR_074542 | 99.00 | ST3-12 | S. spinoverrucosus 174464 | EU593714 | 99.05 | ||
| Kocuria | ST1-1-1-24-2 | K. gwangalliensis SJ2 | NR_116266 | 99.93 | ST2SX7 | S. spinoverrucosus NBRC 14228 | NR_041159 | 97.81 | ||
| ST3-24-1-2 | K. indica SJU27 | MN511772 | 99.86 | ST3SX-4 | S. spinoverrucosus Ng2-6 | MK519101 | 98.62 | |||
| ST2LB-1 | K. rosea 5 | KF923415 | 99.78 | ST1-1-2-9 | S. sp. NO8 | KC200022 | 100.00 | |||
| Lechevalieria | ST1SX-6-2 | L. atacamensis 41-HR6 | MF077035 | 99.46 | ST2-5 | S. xinghaiensis S15077 | MG563223 | 99.78 | ||
| ST2SX-6 | L. atacamensis C61 | NR_116354 | 95.81 | Saccharothrix | ST2B-10-4 | S. lopnurensis YIM LPA2h | NR_145947 | 98.62 | ||
| ST1SX-10 | L. xinjiangensis R24 | NR_044009 | 99.26 | ST3-3-1 | S. yanglingensis Hhs.015 | NR_117283 | 99.55 | |||
| Microbacterium | ST2LB-3 | M. kitamiense kitami C2 | NR_112042 | 99.71 | Modestobacter | ST3-14 | M. caceresii KNN 45-2b | NR_137398 | 99.56 | |
| ST3-6 | M. oxydans 4-46-1-1-1 | MK425667 | 99.85 | ST3-1-1-13 | M. marinus BC501 | FO203431 | 99.93 | |||
| Pseudonocardia | ST1SX-2 | P. hierapolitana PM2084 | NR_126236 | 99.26 | ||||||
| Deinococcus-Thermus | Deinococcus | ST1SX-4 | D. sp. 4B4 | EU029131 | 97.07 | |||||
| ST1-1-1-9 | D. sp. 4B6 | EU029132 | 99.83 | |||||||
| Firmicutes | Bacillus | ST1-1-1-24-1 | B. amyloliquefaciens HX2016004 | MN176577 | 100.00 | Bacillus | ST1SX-3 | B. atrophaeus XAAS.xj4 | MN187264 | 99.86 |
| ST1-1-1-17 | B. amyloliquefaciens KB-82 | KM269197 | 100.00 | ST2SX-3 | B. atrophaeus Y27 | MK721044 | 100.00 | |||
| ST2SS3-1 | B. atrophaeus HAB_5 | MK310269 | 99.93 | ST1-1-1-9 | B. cereus LH8 | KC248215 | 99.93 | |||
| ST1-1SX-3 | B. atrophaeus MER_TA_30 | KT719435 | 99.93 | ST2-19-2 | B. cereus st2 | MF102134 | 99.93 | |||
| ST1-1-1-17-2 | B. licheniformis D69 | KU922147 | 99.86 | ST1-1-2-6 | B. foraminis CD5 | MK216757 | 99.64 | |||
| ST2-19-1 | B. mojavensis h | MG839268 | 100.00 | ST3-1-1-3-1 | B. halotolerans FJAT-45391 | KY849471 | 100.00 | |||
| ST1SX8-1 | B. paramycoides OOF5 | MH542275 | 99.86 | ST2-26 | B. subtilis GuanMX | MN473282 | 100.00 | |||
| ST1-1-2-13 | B. pumilus D51 | JX293284 | 99.93 | ST3-2 | B. subtilis PSBnR5 | MH257752 | 100.00 | |||
| ST1SX-5 | B. safensis MDL5 | MN493773.1 | 100.00 | ST1-1-1-20 | B. subtilis Sk01A | MH210872 | 99.79 | |||
| ST3-27 | B. safensis YZ1709B01 | MK748241.1 | 99.79 | ST3-5 | B. tequilensis 6MS1 | MK713704 | 97.32 | |||
| ST2-29 | B. simplex ZLynn1000-56 | KY316470 | 99.57 | ST1-1-2-1 | B. vallismortis 70.LE.1 | MN149347 | 99.93 | |||
| ST3SS4-1 | B. subtilis 2/30 | MN435586 | 100.00 | ST2B8 | B. vallismortis Y | MG839261 | 98.57 | |||
| ST3-2-2-12 | B. velezensis N8 | KX588164 | 100.00 | |||||||
| Paenibacillus | ST2-28 | P. harenae NJY-3 | MF101120 | 95.90 | Enterococcus | ST3-2-1-9-3 | E. sp. 79w3 | AB675140 | 94.50 | |
| ST3-24-2 | P. polymyxa RCP6 | GU369972 | 99.63 | Staphylococcus | ST2SS2-1 | S. saprophyticus subsp. saprophyticus zzx27 | KJ009395 | 99.51 | ||
| Proteobacteria | Azospirillum | ST3SX-5 | A. palatum ww10 | EU747318 | 94.87 | Massilia | ST3-20 | M. varians 66-LR14-2 | MF077216 | 99.48 |
| ST2SX5-1 | A. sp. NCCP-699 | LC193946 | 96.99 | Methylorubrum | ST2SX-5-1 | M. pseudosasae IMB16-188 | MG190781 | 100.00 | ||
| Belnapia | ST1SX-20 | B. moabensis CP2C | NR_042371 | 99.20 | Microvirga | ST3-2-1-6 | M. aerilata KBL26 | MG576179 | 98.19 | |
| ST3-2-1-11 | B. rosea CPCC 100156 | NR_109297 | 99.85 | ST3-2-1 | M. aerilata NBRC 106137 | NR_114298 | 100.00 | |||
| Brevundimonas | ST2-2 | B. diminuta 264AG7 | KF836539 | 99.78 | ST3-13 | M. ossetica V5/5K | KX576554 | 98.36 | ||
| ST2-10 | B. diminuta HMS9 | MK696984 | 99.92 | ST3-2-1-10 | M. sp. R491-7 | KX444133 | 98.89 | |||
| ST3SS-7 | B. naejangsanensis 5S3 | KM374767 | 99.69 | Pseudomonas | ST2SS3-2 | P. fluorescens psf14 | MN256400 | 99.58 | ||
| ST3-27-2 | B. vesicularis CX-89 | MH368406 | 99.70 | ST3-2-3-12-1-1 | P. putida YP2 | KP313537 | 100.00 | |||
| Candidimonas | ST1-3-2-5 | C. bauzanensis BZ59 | NR_108569 | 98.23 | ST1-1-2-2-1 | P. stutzeri SYJ1-8 | KR262851 | 99.57 | ||
| Enterobacter | ST1SX-6-1 | E. hormaechei SCEH020042 chromosome | CP028538 | 99.71 | Roseomonas | ST3-1-1-14 | R. oryzae JC288 | NR_137403 | 98.34 | |
| Herbaspirillum | ST3-2-1-12 | H. sp. 1NM-18 | JQ608328 | 97.76 | Stenotrophomonas | ST3-3-1-2 | S. rhizophila EGE-B-6 | KP050794 | 99.71 |
表1 河西走廊黑戈壁土壤可培养细菌菌株
Table 1 Culturable bacteria strains isolated from soil in black gobi of the Hexi Corridor
| 门 | 属 | 编号 | 相似菌株 | 登录号 | 相似度/% | 属 | 编号 | 相似菌株 | 登录号 | 相似度/% |
|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Agrococcus | ST2LB-2 | A. citreus ZBGKL11 | KJ734880 | 99.71 | Streptomyces | ST2SX-8 | S. africanus E3SQ | MH472998 | 98.80 |
| ST1-1-2-2 | A. jenensis DW414 | KR856324 | 97.32 | ST1-1-2-5 | S. cacaoi Ru87 | KY818662 | 97.87 | |||
| ST3-6 | A. jenensis Y25 | MK721042 | 99.34 | ST3-3-2-5 | S. capoamus JCM 4734 | NR_040856 | 99.56 | |||
| ST3-4 | A. jenensis Y14 | MK721034 | 99.26 | ST3-3-2-6-1 | S. chryseus HBUM174847 | EU841613 | 97.51 | |||
| ST3-3 | A. terreus BT116 | MH934923 | 99.85 | ST2-S1 | S. coeruleoaurantiacus K7 | KR023963 | 99.85 | |||
| Amycolatopsis | ST3-18 | A. nigrescens CSC17Ta-90 | NR_043880 | 97.93 | ST1-1-1-13 | S. coeruleofuscus MR-18 | KY753217 | 99.85 | ||
| Arsenicicoccus | ST1-1-1-17-1 | A. bolidensis CCUG 47306 | NR_025598 | 99.93 | ST1SX-8 | S. glaucescens NRRL B-2706 | NR_115773 | 99.93 | ||
| Arthrobacter | ST3-16 | A. agilis IHBB 11164 | KR085842 | 99.01 | ST1SX-14 | S. gobitricini LMG 19910 | AJ781335 | 100.00 | ||
| ST3-15 | A. agilis II/11 | KM036066 | 99.71 | ST3-2-1-10-2 | S. lavendulocolor NBRC 12881 | NR_112317 | 99.93 | |||
| ST1-1-2-7 | A. crystallopoietes MR-15 | KY753214 | 99.93 | ST3B1 | S. litmocidini HBUM175011 | FJ486428 | 98.65 | |||
| Auraticoccus | ST3-10 | A. monumenti AL12 | KU258209 | 98.17 | ST1-1-2-4 | S. lunaelactis 244-HNR7 | MF077012 | 99.11 | ||
| ST3-2 | A. monumenti MON 2.2 | LT629688 | 99.55 | ST3-24-1-1 | S. lunaelactis MM109 | CP026304 | 99.12 | |||
| ST3-21 | A. sp. R-68201 | KY386505 | 95.24 | ST1-1-2-3 | S. lunaelactis MMun143 | MG980181 | 99.19 | |||
| ST3-22 | A. monumenti AL12 | KU258209 | 94.31 | ST3-2-1-1-2-1 | S. misionensis 12-4 | KJ571074 | 99.49 | |||
| Blastococcus | ST1-1-1-16 | B. aggregatus 1P10AnA | EU977831 | 99.63 | ST3-2-1-8-1 | S. misionensis cfcc3147 | FJ792563 | 99.63 | ||
| ST3-5-2 | B. capsensis R9 | MK696404 | 99.63 | ST1-1-2-10-1 | S. palmae CMU-AB199 | LC413945 | 98.02 | |||
| ST3-24-1-3 | B. capsensis RQ2 | MK696394 | 99.09 | ST1-1-2-10 | S. palmae CMU-AB204 | NR_152026 | 97.47 | |||
| ST2B4 | B. endophyticus YIM 68236 | NR_108608 | 99.32 | ST2SB-6 | S. piloviolofuscus 174468 | EU593715 | 97.66 | |||
| ST3-12-1 | B. saxobsidens DD2 | FO117623 | 99.78 | ST3-2-2-2 | S. pulveraceus MR-27 | KY753226 | 96.65 | |||
| ST3-24 | B. saxobsidens BC444 | NR_117019 | 99.33 | ST3-3-2-6 | S. sp. Z594b | MN371360 | 97.72 | |||
| ST2SX-1 | B. saxobsidens BC448 | NR_025482 | 99.64 | ST2SX-5-2 | S. rimosus PSK5-20B | MN421096 | 96.81 | |||
| Crossiella | ST3SX-11 | C. equi NRRL B-24104 | NR_025088 | 99.18 | ST1-3-2-2 | S. sioyaensis W24 | KP718602 | 99.12 | ||
| Janibacter | ST1-1-2-11 | J. terrae CS12 | NR_036868 | 99.85 | ST3-2-1-9-1 | S. spinoverrucosus 173372 | EU570683 | 98.89 | ||
| Kineococcus | ST2SS-1 | K. radiotolerans SRS30216 | NR_074542 | 99.00 | ST3-12 | S. spinoverrucosus 174464 | EU593714 | 99.05 | ||
| Kocuria | ST1-1-1-24-2 | K. gwangalliensis SJ2 | NR_116266 | 99.93 | ST2SX7 | S. spinoverrucosus NBRC 14228 | NR_041159 | 97.81 | ||
| ST3-24-1-2 | K. indica SJU27 | MN511772 | 99.86 | ST3SX-4 | S. spinoverrucosus Ng2-6 | MK519101 | 98.62 | |||
| ST2LB-1 | K. rosea 5 | KF923415 | 99.78 | ST1-1-2-9 | S. sp. NO8 | KC200022 | 100.00 | |||
| Lechevalieria | ST1SX-6-2 | L. atacamensis 41-HR6 | MF077035 | 99.46 | ST2-5 | S. xinghaiensis S15077 | MG563223 | 99.78 | ||
| ST2SX-6 | L. atacamensis C61 | NR_116354 | 95.81 | Saccharothrix | ST2B-10-4 | S. lopnurensis YIM LPA2h | NR_145947 | 98.62 | ||
| ST1SX-10 | L. xinjiangensis R24 | NR_044009 | 99.26 | ST3-3-1 | S. yanglingensis Hhs.015 | NR_117283 | 99.55 | |||
| Microbacterium | ST2LB-3 | M. kitamiense kitami C2 | NR_112042 | 99.71 | Modestobacter | ST3-14 | M. caceresii KNN 45-2b | NR_137398 | 99.56 | |
| ST3-6 | M. oxydans 4-46-1-1-1 | MK425667 | 99.85 | ST3-1-1-13 | M. marinus BC501 | FO203431 | 99.93 | |||
| Pseudonocardia | ST1SX-2 | P. hierapolitana PM2084 | NR_126236 | 99.26 | ||||||
| Deinococcus-Thermus | Deinococcus | ST1SX-4 | D. sp. 4B4 | EU029131 | 97.07 | |||||
| ST1-1-1-9 | D. sp. 4B6 | EU029132 | 99.83 | |||||||
| Firmicutes | Bacillus | ST1-1-1-24-1 | B. amyloliquefaciens HX2016004 | MN176577 | 100.00 | Bacillus | ST1SX-3 | B. atrophaeus XAAS.xj4 | MN187264 | 99.86 |
| ST1-1-1-17 | B. amyloliquefaciens KB-82 | KM269197 | 100.00 | ST2SX-3 | B. atrophaeus Y27 | MK721044 | 100.00 | |||
| ST2SS3-1 | B. atrophaeus HAB_5 | MK310269 | 99.93 | ST1-1-1-9 | B. cereus LH8 | KC248215 | 99.93 | |||
| ST1-1SX-3 | B. atrophaeus MER_TA_30 | KT719435 | 99.93 | ST2-19-2 | B. cereus st2 | MF102134 | 99.93 | |||
| ST1-1-1-17-2 | B. licheniformis D69 | KU922147 | 99.86 | ST1-1-2-6 | B. foraminis CD5 | MK216757 | 99.64 | |||
| ST2-19-1 | B. mojavensis h | MG839268 | 100.00 | ST3-1-1-3-1 | B. halotolerans FJAT-45391 | KY849471 | 100.00 | |||
| ST1SX8-1 | B. paramycoides OOF5 | MH542275 | 99.86 | ST2-26 | B. subtilis GuanMX | MN473282 | 100.00 | |||
| ST1-1-2-13 | B. pumilus D51 | JX293284 | 99.93 | ST3-2 | B. subtilis PSBnR5 | MH257752 | 100.00 | |||
| ST1SX-5 | B. safensis MDL5 | MN493773.1 | 100.00 | ST1-1-1-20 | B. subtilis Sk01A | MH210872 | 99.79 | |||
| ST3-27 | B. safensis YZ1709B01 | MK748241.1 | 99.79 | ST3-5 | B. tequilensis 6MS1 | MK713704 | 97.32 | |||
| ST2-29 | B. simplex ZLynn1000-56 | KY316470 | 99.57 | ST1-1-2-1 | B. vallismortis 70.LE.1 | MN149347 | 99.93 | |||
| ST3SS4-1 | B. subtilis 2/30 | MN435586 | 100.00 | ST2B8 | B. vallismortis Y | MG839261 | 98.57 | |||
| ST3-2-2-12 | B. velezensis N8 | KX588164 | 100.00 | |||||||
| Paenibacillus | ST2-28 | P. harenae NJY-3 | MF101120 | 95.90 | Enterococcus | ST3-2-1-9-3 | E. sp. 79w3 | AB675140 | 94.50 | |
| ST3-24-2 | P. polymyxa RCP6 | GU369972 | 99.63 | Staphylococcus | ST2SS2-1 | S. saprophyticus subsp. saprophyticus zzx27 | KJ009395 | 99.51 | ||
| Proteobacteria | Azospirillum | ST3SX-5 | A. palatum ww10 | EU747318 | 94.87 | Massilia | ST3-20 | M. varians 66-LR14-2 | MF077216 | 99.48 |
| ST2SX5-1 | A. sp. NCCP-699 | LC193946 | 96.99 | Methylorubrum | ST2SX-5-1 | M. pseudosasae IMB16-188 | MG190781 | 100.00 | ||
| Belnapia | ST1SX-20 | B. moabensis CP2C | NR_042371 | 99.20 | Microvirga | ST3-2-1-6 | M. aerilata KBL26 | MG576179 | 98.19 | |
| ST3-2-1-11 | B. rosea CPCC 100156 | NR_109297 | 99.85 | ST3-2-1 | M. aerilata NBRC 106137 | NR_114298 | 100.00 | |||
| Brevundimonas | ST2-2 | B. diminuta 264AG7 | KF836539 | 99.78 | ST3-13 | M. ossetica V5/5K | KX576554 | 98.36 | ||
| ST2-10 | B. diminuta HMS9 | MK696984 | 99.92 | ST3-2-1-10 | M. sp. R491-7 | KX444133 | 98.89 | |||
| ST3SS-7 | B. naejangsanensis 5S3 | KM374767 | 99.69 | Pseudomonas | ST2SS3-2 | P. fluorescens psf14 | MN256400 | 99.58 | ||
| ST3-27-2 | B. vesicularis CX-89 | MH368406 | 99.70 | ST3-2-3-12-1-1 | P. putida YP2 | KP313537 | 100.00 | |||
| Candidimonas | ST1-3-2-5 | C. bauzanensis BZ59 | NR_108569 | 98.23 | ST1-1-2-2-1 | P. stutzeri SYJ1-8 | KR262851 | 99.57 | ||
| Enterobacter | ST1SX-6-1 | E. hormaechei SCEH020042 chromosome | CP028538 | 99.71 | Roseomonas | ST3-1-1-14 | R. oryzae JC288 | NR_137403 | 98.34 | |
| Herbaspirillum | ST3-2-1-12 | H. sp. 1NM-18 | JQ608328 | 97.76 | Stenotrophomonas | ST3-3-1-2 | S. rhizophila EGE-B-6 | KP050794 | 99.71 |
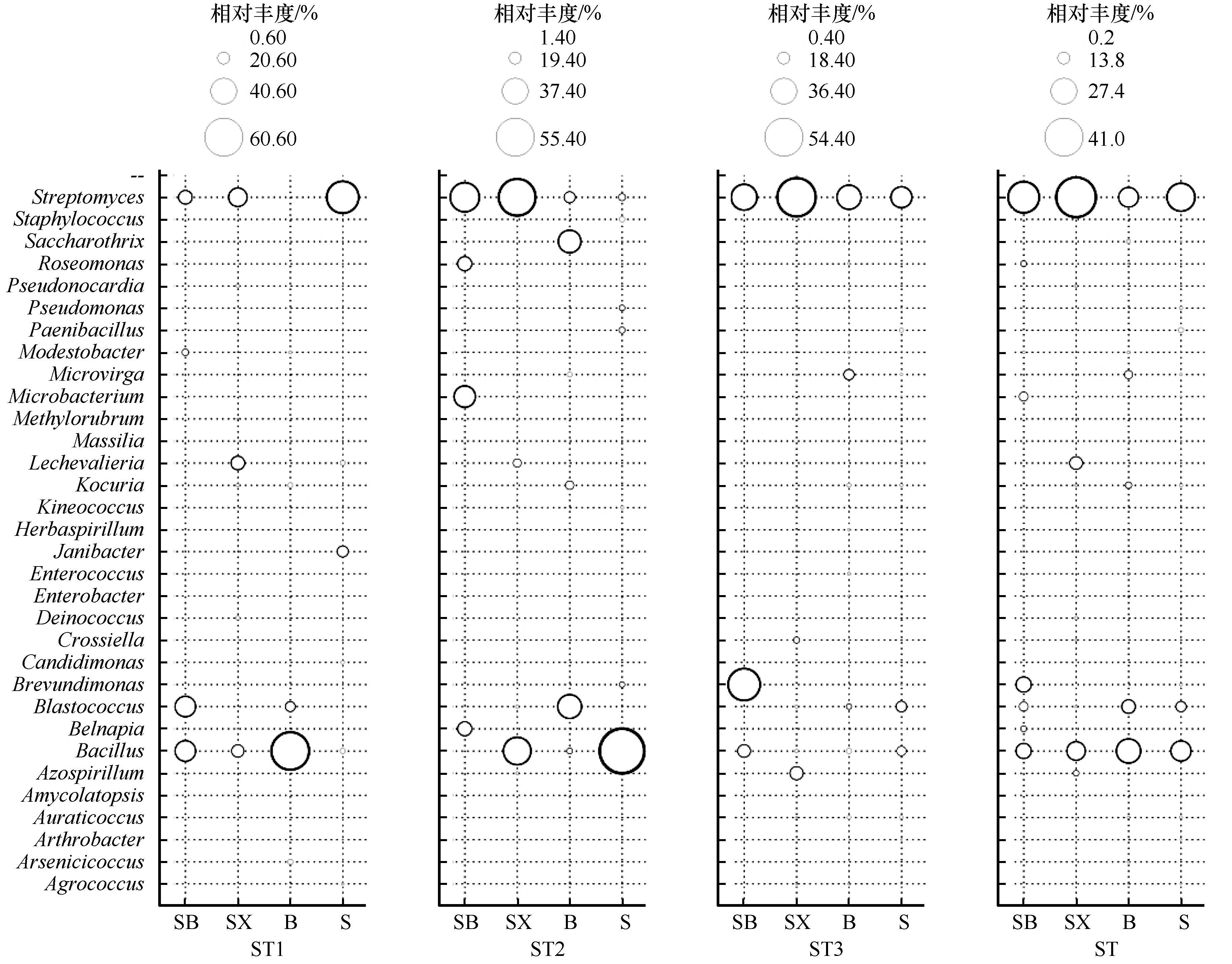
图4 河西走廊黑戈壁土壤菌株相对丰度气泡图SB:砾石上表面土;SX:砾石石下土;B:砾石间隙土;S:深层土
Fig.4 Relative abundance bubble diagram of culturable bacteria strains in black gobi of the Hexi Corridor
| 土壤生境 | Shannon多样性指数 | Simpson多样性指数 |
|---|---|---|
| 砾石上表面土 | 2.3804 | 0.1009 |
| 砾石石下土 | 3.2668 | 0.0518 |
| 砾石间隙土 | 3.4482 | 0.0379 |
| 深层土 | 3.8591 | 0.0253 |
表2 河西走廊黑戈壁可培养细菌多样性指数
Table 2 Species diversity indexes of culturable bacteria in black gobi of the Hexi Corridor
| 土壤生境 | Shannon多样性指数 | Simpson多样性指数 |
|---|---|---|
| 砾石上表面土 | 2.3804 | 0.1009 |
| 砾石石下土 | 3.2668 | 0.0518 |
| 砾石间隙土 | 3.4482 | 0.0379 |
| 深层土 | 3.8591 | 0.0253 |
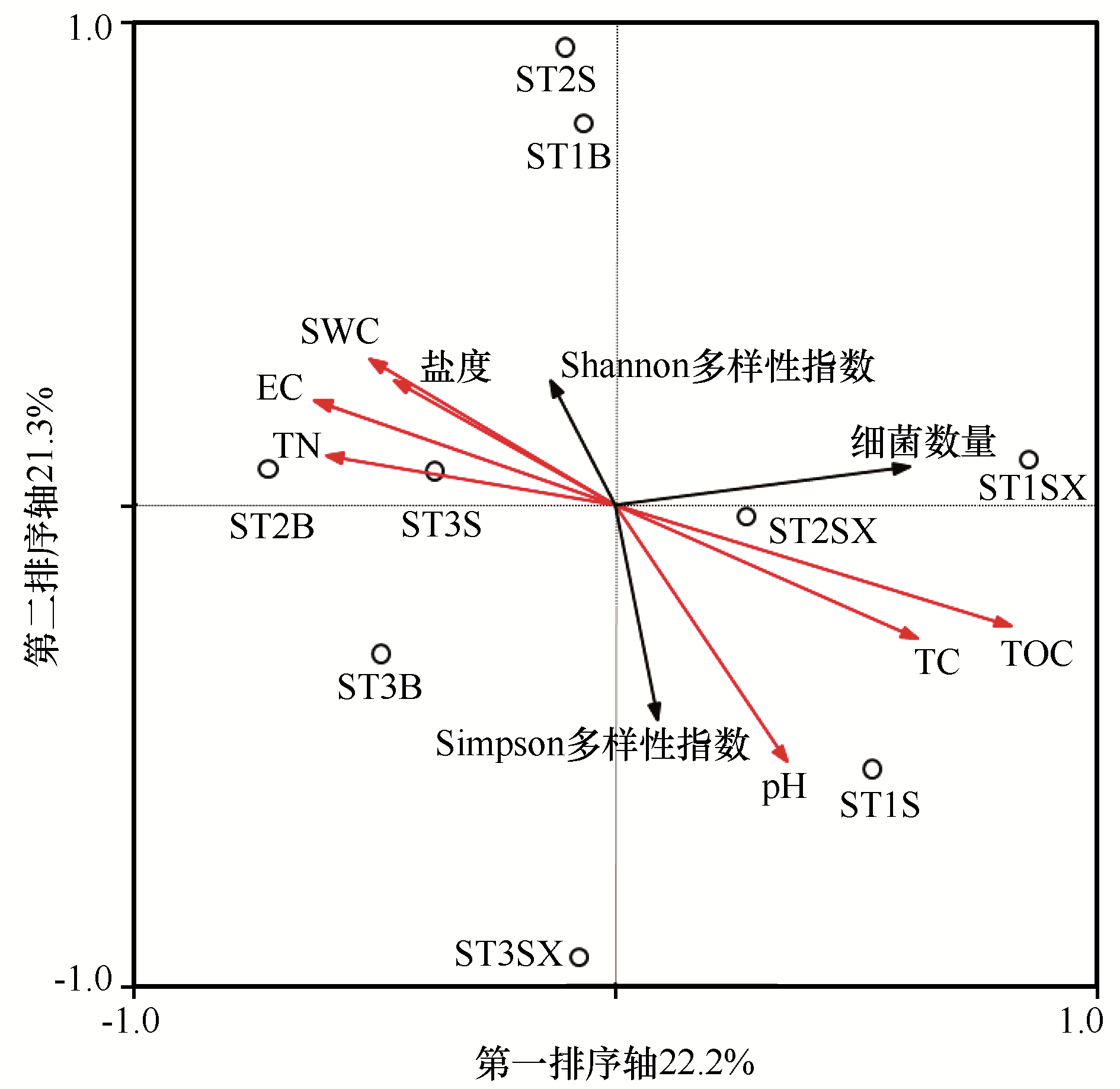
图5 河西走廊黑戈壁可培养细菌和土壤理化因子之间的典型对应分析(CCA)SB:砾石上表面土;SX:砾石石下土;B:砾石间隙土;S:深层土
Fig.5 Typical correspondence analysis (CCA) between soil factors and culturable bacteria in black gobi of the Hexi Corridor
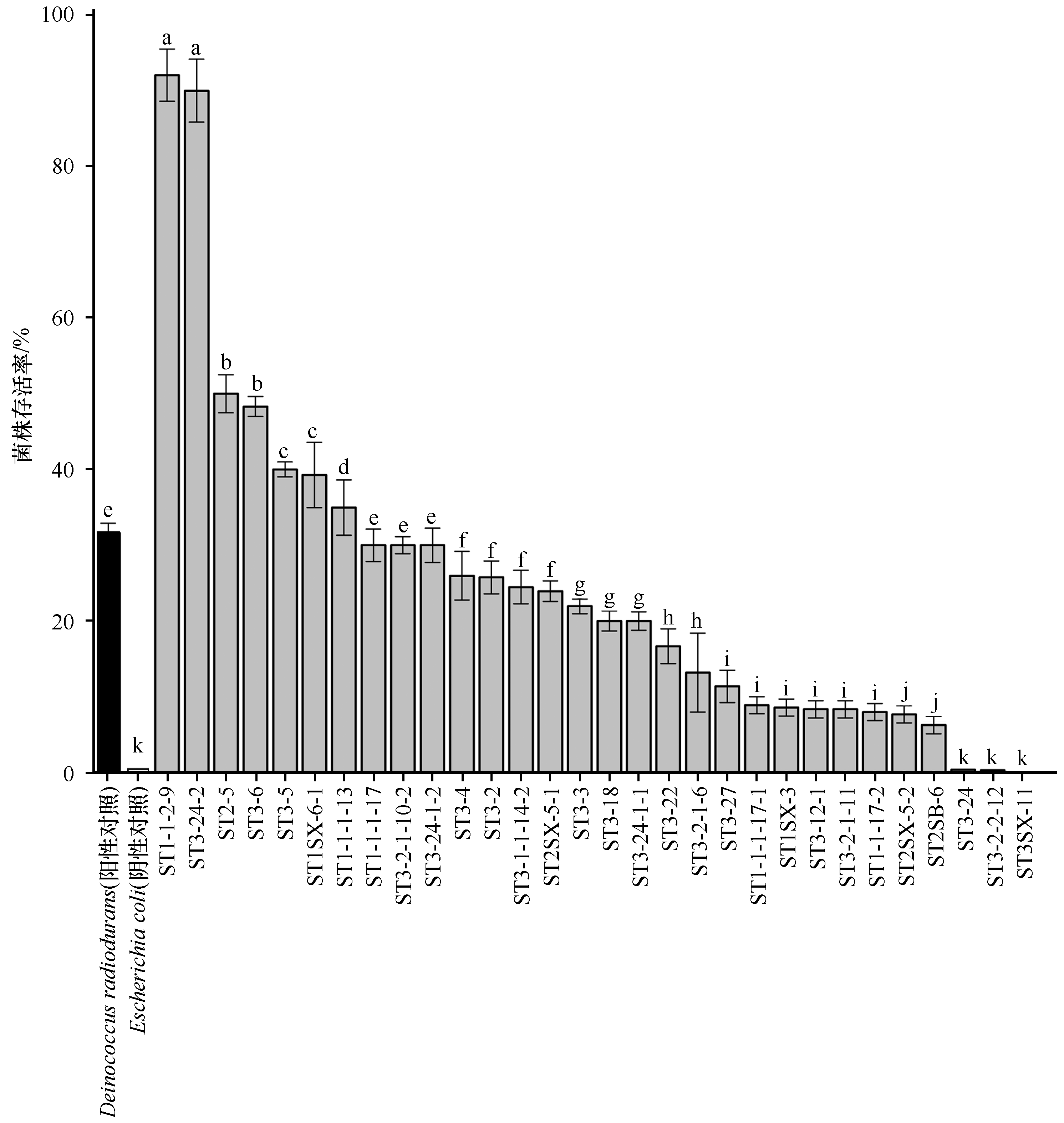
图6 河西走廊黑戈壁部分可培养菌株抗辐射存活率(耐辐射奇球菌(Deinocccus radiodurans)作为阳性对照,大肠杆菌(Escherichia coli)作为阴性对照)不同小写字母表示差异显著(P<0.05),相同小写字母表示差异不显著
Fig.6 Radiation-tolerant survival rate of culturable strains in black gobi of the Hexi Corridor
| 1 | 刘光琇.极端环境微生物学[M].北京:科学出版社,2016:112-138. |
| 2 | 陈永胜.沙漠化土地治理中土壤微生物对生物结皮作用的研究[D].呼和浩特:内蒙古师范大学,2007. |
| 3 | 孔剑捷,陈萍,黄顺心,等.微生物矿化与植物共同作用下荒漠风积沙固化试验研究[J].浙江理工大学学报(自然科学版),2019,41(5):688-696. |
| 4 | 夏延国,宁宇,李景文,等.中国黑戈壁地区植物区系及其物种多样性研究[J].西北植物学报,2013(9):196-205. |
| 5 | 王健铭,董芳宇,巴海·那斯拉,等.中国黑戈壁植物多样性分布格局及其影响因素[J].生态学报,2016(12):3488-3498. |
| 6 | 王晓蕾,张琇,周云锋,等.沙漠微生物群落功能多样性分析[J].水土保持通报,2012,32(3):91-95. |
| 7 | 中国黑戈壁地区生态本底科学考察队.中国黑戈壁研究[M].北京:科学出版社,2014. |
| 8 | 咸迪,郑新江,李雪.中国黑戈壁地区气候变化特征[J].气象与环境学报,2014,30(2):81-87. |
| 9 | 王学全.中国黑戈壁地区水文和水化学调查研究[J].人民黄河,2014,36(9):80-82. |
| 10 | 李雪,郑新江,咸迪,等.中国黑戈壁地区日照时数时空变化及影响因素[J].干旱气象,2013(3):24-28. |
| 11 | Gleason K E,McConnell J R,Arienzo M M,et al.Four-fold increase in solar forcing on snow in western U.S.burned forests since 1999[J].Nature Communication,2019,10(1):2026. |
| 12 | 李婷,张威,刘光琇,等.荒漠土壤微生物群落结构特征研究进展[J].中国沙漠,2018,38(2):329-338. |
| 13 | Navarro-González R,Rainey F A,Molina P,et al.Mars-like soils in the Atacama Desert,Chile,and the dry limit of microbial life[J].Science,2003,302(5647):1018-1021. |
| 14 | Cameron R E,Kink J,David C N,et al.Microbiology,ecology and microclimatology of soil sites in dry valleys of southern Victoria Land,Antarctica[M]//Holdgate M W.Antarctic Ecology.London,UK:Academic Press,1970:702-716. |
| 15 | Martina K,Henry M,Elshahat M,et al.Desert farming benefits from microbial potential in arid soils and promotes diversity and plant health[J].Plos One,2011,6(9):e24452. |
| 16 | Schlesinger W H,Pippen J S,Wallenstein M D,et al.Community composition and photosynthesis by photoautotrophs under Quartz Pebbles,Southern Mojave Desert[J].Ecology,2003,84(12):3222-3231. |
| 17 | Berner T,Evenari M.The influence of temperature and light penetration on the abundance of the hypolithic algae in the Negev desert of Israel[J].Oecologia,1978,33(2):255-260. |
| 18 | Büdel B,Lange O.Water status of green and blue-green phycobionts in Lichen Thalli after hydration by water vapor uptake:Do they become turgid[J].Botanica Acta,1991,104(5):361-366. |
| 19 | Smith M C,Bowman J P,Scott F J,et al.Sublithic bacteria associated with Antarctic quartz stones[J].Antarctic Science,2000,12(2):177-184. |
| 20 | 吴明辉,章高森,陈拓,等.石生微生物研究进展[J].微生物学杂志,2017,37(4):64-73. |
| 21 | Wu N,Zhang Y M,Pan H X,et al.The role of nonphotosynthetic microbes in the recovery of biological soil crusts in the Gurbantunggut Desert,Northwestern China[J].Arid Soil Research and Rehabilitation,2010,24(1):15. |
| 22 | An S,Couteau C,Luo F,et al.Bacterial diversity of surface sand samples from the Gobi and Taklamaken Deserts[J].Microbial Ecology,2013,66(4):850-860. |
| 23 | Sterflinger K,Tesei D,Zakharova K.Fungi in hot and cold deserts with particular reference to microcolonial fungi[J].Fungal Ecology,2012,5(4):453-462. |
| 24 | Grishkan I,Nevo E.Spatiotemporal distribution of soil microfungi in the Makhtesh Ramon area,central Negev desert,Israel[J].Fungal Ecology,2010,3(4):326-337. |
| 25 | Yadav A N,Sachan S G,Verma P,et al.Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes[J].Journal of Bioscience and Bioengineering,2015,119(6):683-693. |
| 26 | Orlando J,Alfaro M,Bravo L,et al.Bacterial diversity and occurrence of ammonia-oxidizing bacteria in the Atacama Desert soil during a “desert bloom” event[J].Soil Biology and Biochemistry,2010,42(7):1183-1188. |
| 27 | Zhang W,Zhang G S,Liu Gx,et al.Bacterial diversity and distribution in the southeast edge of the Tengger Desert and their correlation with soil enzyme activities[J].Journal of Environmental Sciences,2012,24(11):2004-2011. |
| 28 | 吕星宇,张志山.固沙植被区土壤质地与土壤微生物数量的关系[J].中国沙漠,2019,39(5):71-79. |
| 29 | Goswami D,Pithwa S,Dhandhukia P,et al.Delineating Kocuria turfanensis 2M4 as a credible PGPR:a novel IAA-producing bacteria isolated from saline desert[J].Journal of Plant Interactions,2014,9(1):566-576. |
| 30 | 刘少芳,王若愚.植物根际促生细菌提高植物耐盐性研究进展[J].中国沙漠,2019,39(2):1-12. |
| 31 | Lester E D,Satomi M,Ponce A.Microflora of extreme arid Atacama Desert soils[J].Soil Biology and Biochemistry,2007,39(2):704-708. |
| 32 | Andrew D R,Fitak R R,Munguia-Vega A,et al.Abiotic factors shape microbial diversity in Sonoran Desert soils[J].Applied Environmental Microbiology,2012,78(21):7527-7537. |
| 33 | Yu L Z,Luo X S,Liu M,et al.Diversity of ionizing radiation-resistant bacteria obtained from the Taklimakan Desert[J].Journal of Basic Microbiology,2015,55(1):135-140. |
| 34 | Goodfellow M,Busarakam K,Idris H,et al.Streptomyces asenjonii sp.nov.,isolated from hyper-arid Atacama Desert soils and emended description of Streptomyces viridosporus Pridhamet al.1958[J].Antonie Van Leeuwenhoek,2017,110:1133-1148. |
| 35 | Chanal A,Chapon V,Benzerara K,et al.The desert of Tataouine:an extreme environment that hosts a wide diversity of microorganisms and radiotolerant bacteria[J].Environmental Microbiology,2006,8(3):514-525. |
| 36 | Prestel E,Salamitou S,Dubow M S.An examination of the bacteriophages and bacteria of the Namib desert[J].Journal of Microbiology,2008,46(4):364. |
| 37 | Winsley T,van Dorst J M,Brown M V,et al.Capturing greater 16S rRNA gene sequence diversity within the domain Bacteria[J].Applied Environmental Microbiology,2012,78(16):5938-5941. |
| 38 | Holmes A J,Bowyer J,Holley M P,et al.Diverse,yet-to-be-cultured members of the Rubrobacter subdivision of the Actinobacteria are widespread in Australian arid soils[J].FEMS Microbiology Ecology,2000,33(2):111-120. |
| 39 | 申枚灵,赵翀,廖萍,等.塔里木盆地光果甘草内生放线菌的分离鉴定及抗逆、促生特性[J].草业科学,2018,35(7):1624-1633. |
| 40 | 梁亚萍,宗兆锋,马强.6株野生植物内生放线菌防病促生作用的初步研究[J].西北农林科技大学学报(自然科学版),2017,35(7):131-136. |
| 41 | 杨雅琳,赵翀,廖萍,等.塔里木盆地胀果甘草内生放线菌多样性及抗菌活性分析[J].微生物学通报,2016,43(10):2138-2147. |
| 42 | 谢永丽.青海柴达木极端干旱沙地分离芽孢杆菌的分子鉴定及拮抗活性分析[J].微生物学通报,2012,39(8):1079-1086. |
| 43 | 马欣,刘俊,乔俊卿,等.利用转座子 TnYLB-1 构建枯草芽孢杆菌的突变体文库[J].南京农业大学学报,2011,34(6):77-81. |
| 44 | 廖畅,田秋香,汪东亚,等.外源碳输入对中亚热带森林深层土壤碳矿化和微生物决策群落的影响[J].应用生态学报,2016,27(9):2848-2854. |
| 45 | 胡华英,殷丹阳,周垂帆.生物炭对杉木人工林土壤磷素有效性的影响机制[C]//中国土壤学会土壤环境专业委员会第二十次会议暨农田土壤污染与修复研讨会论文集.2018. |
| 46 | 王佩雯,朱金峰,陈征,等.高通量测序技术下连作植烟土壤细菌群落与土壤环境因子的耦合分析[J].农业生物技术学报,2016,24(11):1754-1763. |
| 47 | 姚钦.生物炭施用对东北黑土土壤理化性质和微生物多样性的影响[D].长春:中国科学院东北地理与农业生态研究所,2017. |
| 48 | Gonçalves V N,Cantrell C L,Wedge D E,et al.Fungi associated with rocks of the Atacama Desert:taxonomy,distribution,diversity,ecology and bioprospection for bioactive compounds[J].Environmental Microbiology,2016,18(1):232-245. |
| 49 | 谢自力,张荣,修向前,等.用于紫外探测器DBR结构的高质量AlGaN材料MOCVD生长及其特性研究[J].物理学报,2007(11):6717-6721. |
| 50 | 邓小宁.紫外线杀菌作用的研究[J].中国照明电器,1994(6):39-41. |
| 51 | 代芳平,李师翁.链霉菌次级代谢物及其应用研究进展[J].生物技术通报,2014(3):30-35. |
| 52 | Wilson Z E,Brimble M A.Molecules derived from the extremes of life[J].Natural Product Reports,2009,26(1):44-71. |
| 53 | 李生樟,陈颖,杨瑞环,等.一株拮抗黄单胞菌的贝莱斯芽孢杆菌的分离和鉴定[J].微生物学报,2019,59(10):1969-1983. |
| [1] | 张立彭, 师桂英, 史贵红, 于彦琳, 李谋强, 苏国礼, 贾喜霞. 土壤熏蒸-微生物菌剂联用缓解兰州百合(Lilium davidii var. unicolor)连作障碍研究[J]. 中国沙漠, 2020, 40(5): 169-179. |
| [2] | 苏天燕, 刘文杰, 杨秋, 毛伟. 土壤碳循环对地下水位的响应研究进展[J]. 中国沙漠, 2020, 40(5): 180-189. |
| [3] | 马晓俊, 李云飞. 腾格里沙漠东南缘植被恢复过程中土壤微生物量及酶活性[J]. 中国沙漠, 2019, 39(6): 159-166. |
| [4] | 吕星宇, 张志山. 固沙植被区土壤质地与土壤微生物数量的关系[J]. 中国沙漠, 2019, 39(5): 71-79. |
| [5] | 范虹, 赵财, 胡发龙, 肖凯, 柴强. 土壤含水量、种植模式和丁香酚对土壤微生物种群结构的影响[J]. 中国沙漠, 2019, 39(2): 13-18. |
| [6] | 杨航宇, 刘长仲, 刘艳梅, 杨昊天. 荒漠区踩踏生物土壤结皮对土壤微生物量的影响[J]. 中国沙漠, 2019, 39(2): 35-44. |
| [7] | 李婷, 张威, 刘光琇, 陈拓. 荒漠土壤微生物群落结构特征研究进展[J]. 中国沙漠, 2018, 38(2): 329-338. |
| [8] | 杨航宇, 刘艳梅, 王廷璞, 回嵘. 生物土壤结皮对荒漠区土壤微生物数量和活性的影响[J]. 中国沙漠, 2017, 37(5): 950-960. |
| [9] | 石万里, 王辉, 马维伟. 沙区植被恢复对土壤微生物量及活性的影响[J]. 中国沙漠, 2017, 37(3): 507-513. |
| [10] | 安晶, 吴楠, 张元明. 沙土灭菌对羽毛针禾(Stipagrostis pennata)种子萌发、幼苗生长及根鞘形成的影响[J]. 中国沙漠, 2016, 36(2): 399-405. |
| [11] | 王晓朦, 乌云娜, 宋彦涛, 霍光伟, 徐志超, 道日娜. 放牧对克氏针茅(Stipa krylovii)草原土壤物理、化学及微生物性状的影响[J]. 中国沙漠, 2015, 35(5): 1193-1199. |
| [12] | 袁立敏, 高永, 黄海广, 闫德仁, 胡小龙, 胡志健. 生物模块沙障对流动沙丘土壤微生物分布的影响[J]. 中国沙漠, 2015, 35(1): 189-194. |
| [13] | 柴晓虹, 吴永华, 钟芳. 不同降雨量下人工重建林土壤性状及微生物特性比较[J]. 中国沙漠, 2014, 34(6): 1576-1583. |
| [14] | 钟芳, 柴晓虹, 王国基, 段争虎. 植被恢复方式对黄土丘陵区土壤理化性质及微生物特性的影响[J]. 中国沙漠, 2014, 34(4): 1064-1072. |
| [15] | 马文文, 姚拓, 靳鹏, 王国基, 张玉霞. 荒漠草原2种植物群落土壤微生物及土壤酶特征[J]. 中国沙漠, 2014, 34(1): 176-183. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
©2018中国沙漠 编辑部
地址: 兰州市天水中路8号 (730000)
电话:0931-8267545
Email:caiedit@lzb.ac.cn;desert@lzb.ac.cn
