中国沙漠 ›› 2020, Vol. 40 ›› Issue (4): 128-137.DOI: 10.7522/j.issn.1000-694X.2019.00121
收稿日期:2019-08-23
修回日期:2019-12-24
出版日期:2020-08-20
发布日期:2020-09-01
通讯作者:
于明含
作者简介:于明含(E-mail: ymh_2012tai@163.com)基金资助:
Ziyuan Zhou( ), Minghan Yu(
), Minghan Yu( ), Guodong Ding, Guanglei Gao, Yingying He
), Guodong Ding, Guanglei Gao, Yingying He
Received:2019-08-23
Revised:2019-12-24
Online:2020-08-20
Published:2020-09-01
Contact:
Minghan Yu
摘要:
基于16S rRNA高通量基因测序技术,对毛乌素沙地小叶锦鸡儿(Caragana microphylla)、柠条锦鸡儿(Caragana korshinskii)根系微域(即根系、根际土、根区土、灌丛间空白土)间的细菌群落多样性和结构差异性进行表征。本研究对各根系微域间细菌群落的Alpha多样性指数进行了单因素方差分析以及基于OTU水平的PCA分析,探究其在根系微域间Alpha和Beta多样性的层级变化,证实了有关植物根系微域生态位分化的报道,并发现锦鸡儿属植物根系微域间细菌群落的多样性和结构组成随着4个微域类型由外及内呈现出显著的层级差异性(P<0.05)。通过对优势细菌群组的结构组成分析,发现锦鸡儿属植物对特定细菌群组具有显著的向根系内筛选富集的作用(P<0.05)。这种植物通过根系微域对特定细菌群组的逐级筛选富集作用,是导致锦鸡儿属植物灌丛下不同生态位间细菌群落结构和组成发生层级变异的主要原因。
中图分类号:
周子渊, 于明含, 丁国栋, 高广磊, 何莹莹. 毛乌素沙地锦鸡儿(Caragana)根系微域细菌群落多样性特征[J]. 中国沙漠, 2020, 40(4): 128-137.
Ziyuan Zhou, Minghan Yu, Guodong Ding, Guanglei Gao, Yingying He. Diversity of bacterial communities in the rhizocompartments of Caragana in the Mu Us Desert[J]. Journal of Desert Research, 2020, 40(4): 128-137.
| Alpha多样性指数 | Chao1指数 | Shannon指数 | OTU数目 | Good’s Coverage指数 |
|---|---|---|---|---|
| CM-r | 1 707.11±457.09b | 6.95 ± 0.64b | 1 024±276b | 96.98%±0.86%a |
| CM-rs | 2 790.39±319.51a | 9.44± 0.16a | 2 000±181a | 95.13%±0.74%b |
| CM-rz | 2 735.70±255.00a | 9.30± 0.28a | 1 915±186a | 95.24%±0.53%b |
| Bulk | 2 440.77±413.01a | 8.90± 0.41a | 1 728±278a | 95.76%±0.81%b |
表1 根系微域细菌群落Alpha多样性指数及各分类学水平结构统计(n=5)
Table 1 General features of the high-throughput sequencing results(n = 5)
| Alpha多样性指数 | Chao1指数 | Shannon指数 | OTU数目 | Good’s Coverage指数 |
|---|---|---|---|---|
| CM-r | 1 707.11±457.09b | 6.95 ± 0.64b | 1 024±276b | 96.98%±0.86%a |
| CM-rs | 2 790.39±319.51a | 9.44± 0.16a | 2 000±181a | 95.13%±0.74%b |
| CM-rz | 2 735.70±255.00a | 9.30± 0.28a | 1 915±186a | 95.24%±0.53%b |
| Bulk | 2 440.77±413.01a | 8.90± 0.41a | 1 728±278a | 95.76%±0.81%b |
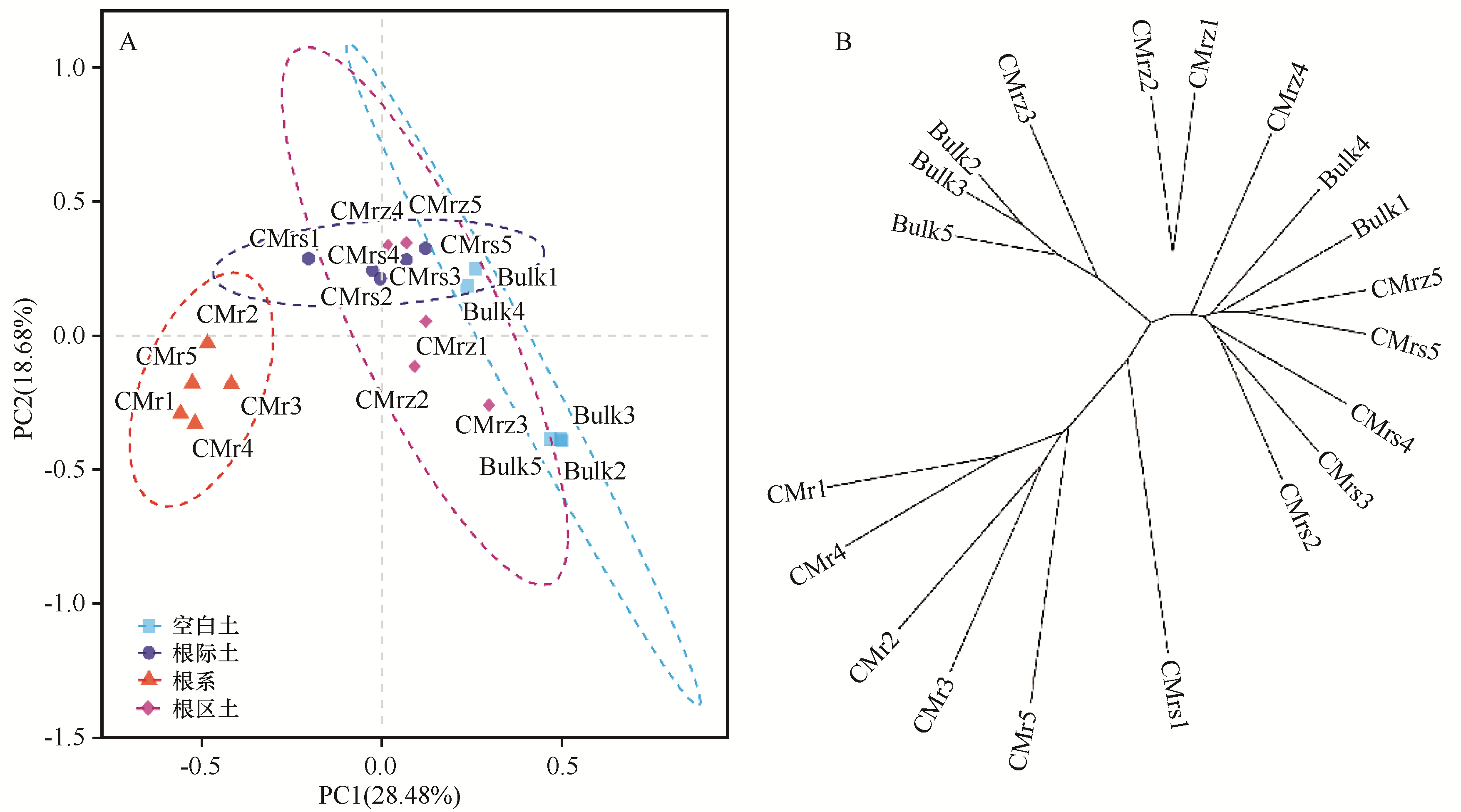
图1 根系微域在OTU水平上驱动着细菌群落的组成A:荒漠豆科锦鸡儿属植物根系微域细菌群落组成主成分PCA分析;B:基于Bray-Curtis差异性的样本层次聚类。CM:锦鸡儿属植物,r:根系,rs:根际土,rz:根区土,Bulk:灌丛间空白土。弧线所包含的样本,相似性水平在95%以上聚类
Fig.1 Rhizocompartment drives the composition of the bacterial communities at the OTU level
| 根系微域 | R | P |
|---|---|---|
| CMr-CMrs | 0.912 | 0.005 |
| CMr-CMrz | 0.974 | 0.010 |
| CMrs-CMrz | 0.100 | 0.200 |
| Bulk-CMr | 1.000 | 0.008 |
| Bulk-CMrs | 0.640 | 0.005 |
| Bulk-CMrz | 0.228 | 0.100 |
| r-rs-rz-Bulk | 0.480 | 0.001 |
表2 4个根系微域样本ANOSIM分析
Table 2 ANOSIM analysis of rhizocompartments
| 根系微域 | R | P |
|---|---|---|
| CMr-CMrs | 0.912 | 0.005 |
| CMr-CMrz | 0.974 | 0.010 |
| CMrs-CMrz | 0.100 | 0.200 |
| Bulk-CMr | 1.000 | 0.008 |
| Bulk-CMrs | 0.640 | 0.005 |
| Bulk-CMrz | 0.228 | 0.100 |
| r-rs-rz-Bulk | 0.480 | 0.001 |
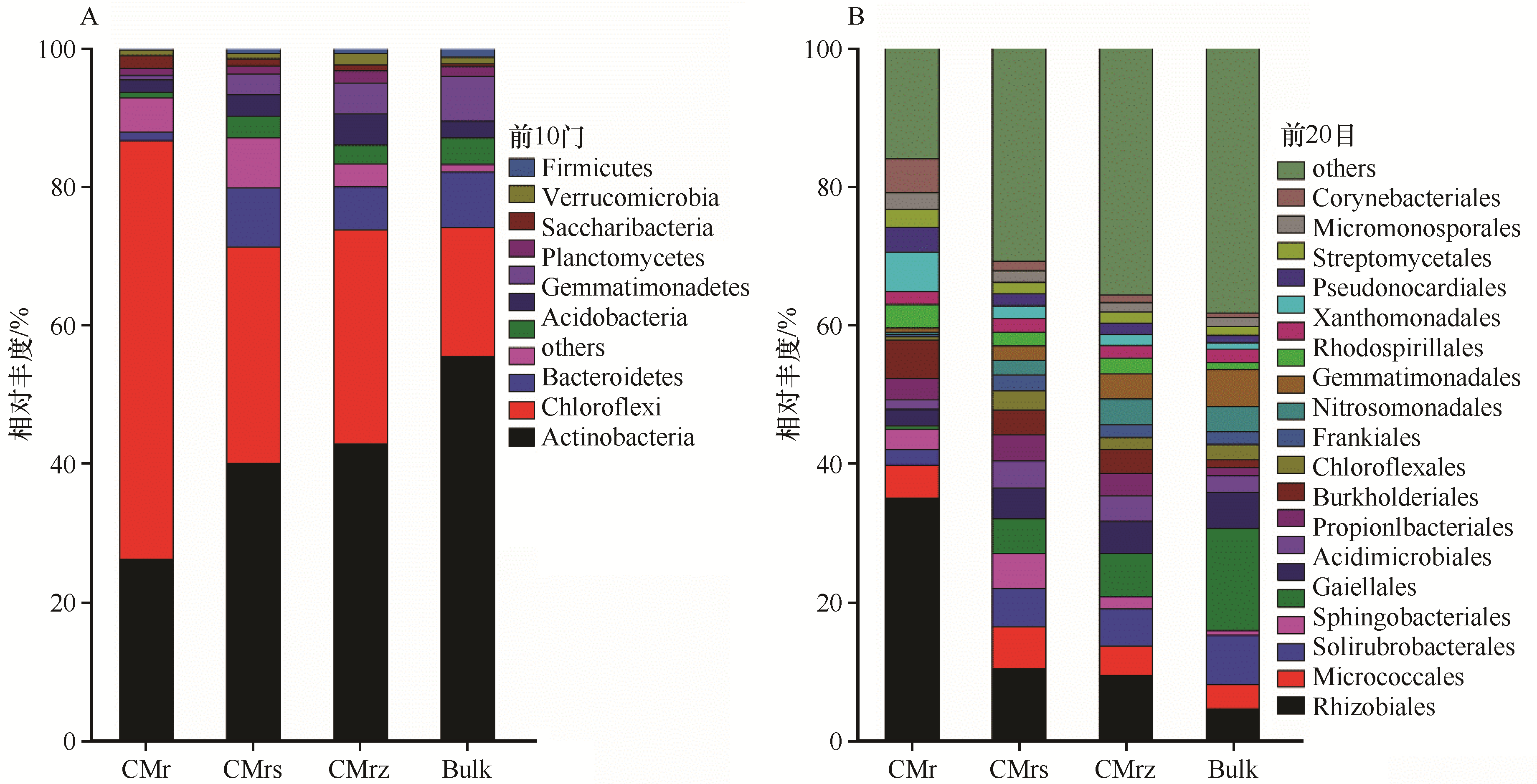
图2 豆科锦鸡儿属植物4个微域微生物群落门-目水平物种组成CM:锦鸡儿属植物,r:根系,rs:根际土,rz:根区土,Bulk:灌丛间空白土;前10以外的门、前20以外的目记为others
Fig.2 Microbial community compositions in the four rhizocompartments of Caragana Fabr. at the phylum and order levels
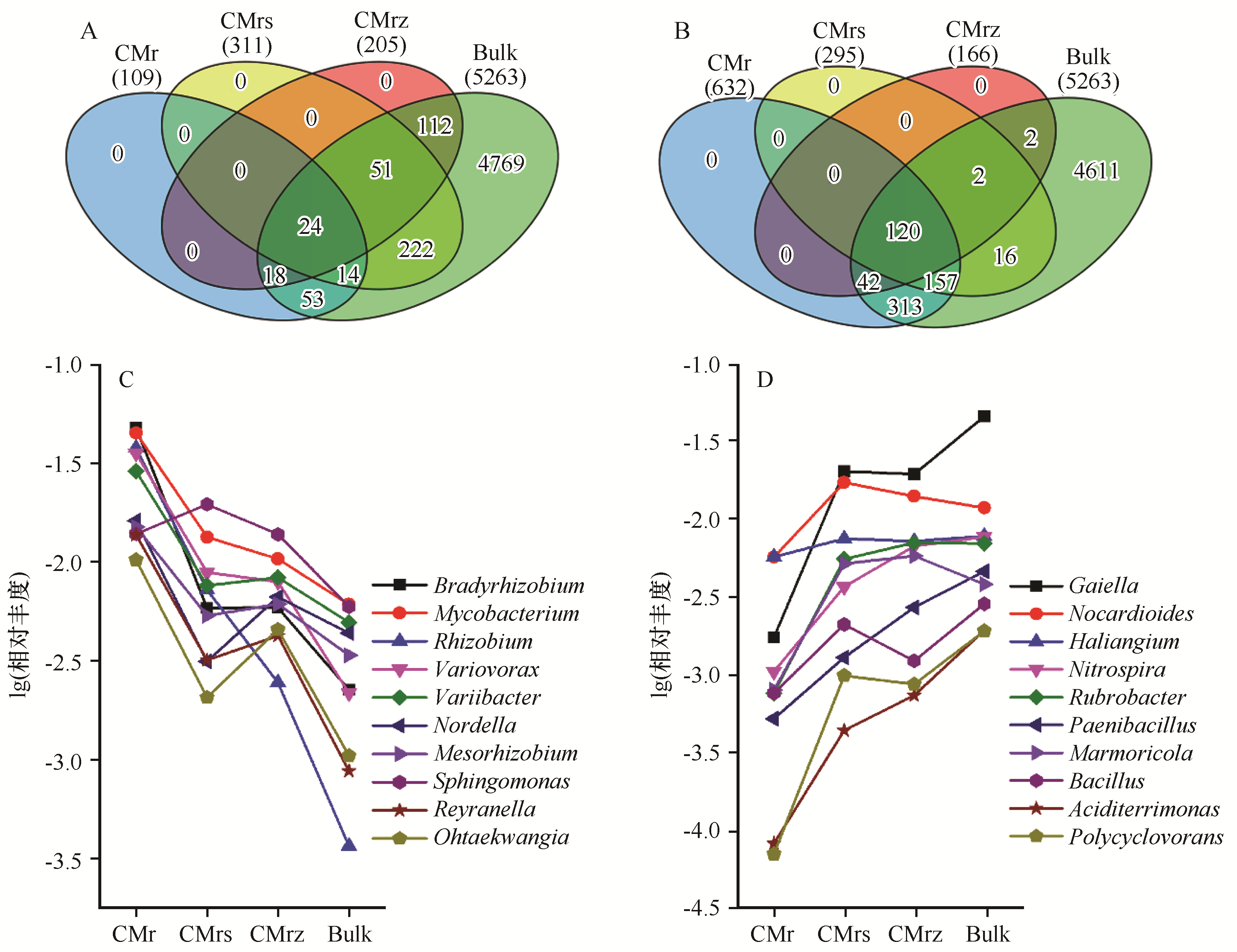
图3 豆科锦鸡儿属植物灌丛下,与根区土壤相比其他3个根系微域OTU相对丰度显著富集(A)和减少(B)的韦恩图,C显示相对富集的OTUs对应的菌属;D显示相对减少的OTUs对应的菌属CM:锦鸡儿属植物,r:根系,rs:根际土,rz:根区土,Bulk:灌丛间空白土
Fig.3 Venn diagrams showing significant enrichment (A) and reduction (B) of the relative operational taxonomic unit (OTU) abundances in the three rhizocompartments compared to root zone soil. C, the bacteria genera corresponding to relatively enriched OTUs; D, the bacteria genera corresponding to relatively reduced OTUs
| 1 | Zhu B B,Li Z B,Li P,et al.Soil erodibility,microbial biomass,and physical-chemical property changes during long-term natural vegetation restoration:a case study in the Loess Plateau, China[J].Ecological Research,2010,25:531-541. |
| 2 | Wang B,Xue S,Liu G B,et al.Changes in soil nutrient and enzyme activities under different vegetations in the Loess Plateau area,northwest China[J].Catena,2012,92:186-195. |
| 3 | 陈文新,王恩涛.中国根瘤菌[M].北京:科学出版社,2011:278-281. |
| 4 | 周道玮,刘钟龄,马毓泉.豆科锦鸡儿属(Caragana Fabr.)植物地理分布与分化研究[J].植物研究,2005,25(4):471-487. |
| 5 | Lai Z R,Zhang Y Q,Liu J B,et al.Fine-root distribution,production,decomposition,and effect on soil organic carbon of three revegetation shrub species in northwest China[J].Forest Ecology and Management,2016,359:381-388. |
| 6 | Pérez-Bejarano A,Mataix-Solera J,Zornoza R,et al.Influence of plant species on physical,chemical and biological soil properties in a Mediterranean forest soil[J].European Journal of Forest Research,2010,129(1):15-24. |
| 7 | Saul-Tcherkas V,Steinberger Y.Soil Microbial diversity in the vicinity of a Negev Desert shrub-Reaumuria negevensis[J].Microbial Ecology,2011,61(1):64-81. |
| 8 | Mengual C,Schoebitz M,Azcón R,et al.Microbial inoculants and organic amendment improves plant establishment and soil rehabilitation under semiarid conditions[J].Journal of Environmental Management,2014,134:1-7. |
| 9 | Berendsen R L,Pieterse C M J,Bakker P A.The rhizosphere microbiome and plant health[J].Trends in Plant Science,2012,17:478-86. |
| 10 | Egamberdieva D,Kamilova F,Validov S,et al.High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan[J].Environment Microbiology,2008,10:1-9. |
| 11 | Mendes R,Kruijt M,De Bruijn I,et al.Deciphering the rhizosphere microbiome for disease-suppressive bacteria[J].Science,2011,332(6033):1097-1100. |
| 12 | Beckers B,Op De Beeck M,Weyens N,et al.Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees[J].Microbiome,2017,5:25. |
| 13 | Li X Z,Rui J P,Mao Y J,et al.Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar[J].Soil Biology and Biochemistry,2014,68,392-401. |
| 14 | Wang Y B,Zhang W X,Ding C J,et al.Endophytic communities of transgenic poplar were determined by the environment and niche rather than by transgenic events[J].Frontiers in Microbiology,2019,26(10):588. |
| 15 | Hoitink H,Boehm M.Biocontrol within the context of soil microbial communities:a substrate-dependent phenomenon[J].Annual Review of Phytopathology,1999,37:427-446. |
| 16 | Millet Y A.Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns[J].Plant Cell,2010,22:973-990. |
| 17 | Hallmann J A,Quadt-Hallmann A,Mahaffee W F,et al.Endophytic bacteria in agricultural crops[J].Canadian Journal of Microbiology,2011,43:895-914. |
| 18 | Hardoim P R,Overbeek V,Leo S,Van J D.Properties of bacterial endophytes and their proposed role in plant growth[J].Trends in Microbiology,2008,16:463-471. |
| 19 | Prischl M,Hackl E,Pastar M,et al.Genetically modified Btmaize lines containing cry3Bb1,cry1A105 or cry1Ab2 do not affect the structure and functioning of root-associated endophyte communities[J].Applied Soil Ecology,2012,54:39-48. |
| 20 | Naveed M,Mitter B,Reichenauer T G,et al.Increased drought stress resilience of maize through endophytic colonization by Burkholderiaphytofirmans PsJN and Enterobacter sp.FD17[J].Environmental and Experimental Botany,2014,97:30-39. |
| 21 | Agler M T,Ruhe J,Kroll S,et al.Microbial hub Taxa link host and abiotic factors to plant microbiome variation[J].PLOS Biology,2016,14(1):1-31. |
| 22 | Bulgarelli D,Garrido-Oter R,Münch P,et al.Structure and function of the bacterial root microbiota in wild and domesticated barley[J].Cell Host Microbe,2015,17:392-403. |
| 23 | Devine T E,Kuykendall L D.Host genetic control of symbiosis in soybean (Glycine max L.)[J].Plant and Soil,1996,186:173-187. |
| 24 | Berg G,Grube M,Schloter M,et al.Unraveling the plant microbiome:looking back and future perspectives[J].Frontiers in Microbiology,2014,5:148. |
| 25 | Barrett L G,Bever J D,Bissett A,et al.Partner diversity and identity impacts on plant productivity in Acacia-rhizobial interactions[J].Journal of Ecology,2015,103:130-142. |
| 26 | Dinnage R,Simonsen A K,Barrett L G,et al.Larger plants promote a greater diversity of symbiotic nitrogen fixing soil bacteria associated with an Australian endemic legume[J].Journal of Ecology,2018,107:977-991. |
| 27 | Gottel N R,Castro H F,Kerley M,et al.Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types[J].Applied and Environmental Microbiology,2011,77:5934-5944. |
| 28 | Lundberg D S,Lebeis S L,Paredes S H,et al.Defining the core Arabidopsis thaliana root microbiome[J].Nature,2012,488:86-90. |
| 29 | Hartmann A,Rothballer M,Schmid M.Lorenz Hiltner,a pioneer in rhizosphere microbial ecology and bacteriology research[J].Plant and Soil,2008,312(1):7-14. |
| 30 | Sun Y,Zhang Y,Feng W,et al.Effects of xeric shrubs on soil microbial communities in a desert in northern china[J].Plant and Soil,2017,414:281-294. |
| 31 | Yu M H,Ding G D,Gao G L,et al.How the plant temperature links to the air temperature in the desert plant Artemisia ordosica[J].PLOS ONE,2015,10(8):e0135452. |
| 32 | Gao G L,Ding G D,Zhao Y Y,et al Fractal approach to estimating changes in soil properties following the establishment of Caragana korshinskii shelterbelts in Ningxia,China NW [J].Ecological Indicators,2014,43:236-243. |
| 33 | Bulgarelli D,Rott M,Schlaeppi K,et al.Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota[J].Nature,2012,488:91-95. |
| 34 | Sánchez-López A S,Thijs S,Beckers B,et al.Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues[J].Plant and Soil,2018,422(1/2):51-66. |
| 35 | Magoč T,Salzberg S L.FLASH:fast length adjustment of short reads to improve genome assemblies[J].Bioinformatics,2011,27:2957-2963. |
| 36 | Edgar R C,Haas B J,Clemente J C,et al.UCHIME improves sensitivity and speed of chimera detection[J].Bioinformatics,2011,27(16):2194-2200. |
| 37 | Edgar R C.UPARSE:highly accurate OTU sequences from microbial amplicon reads[J].Nature Methods,2013,10:996-998. |
| 38 | Chen W X,Li G S,Qi Y L,et al.Rhizobium huakuii sp.nov.isolated from the root nodules of Astragalus sinicus[J].International Journal of Systematic and Evolutionary Microbiology,1991,41:275-280. |
| 39 | Zhang X X,Turner S L,Guo X W,et al.The common nodulation genes of Astragalus sinicus Rhizobia are conserved despite chromosomal diversity[J].Applied and Environmental Microbiology,2000,66(7):2988-2995. |
| 40 | Nemergut D R,Cleveland C C,Wieder W R,et al.Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest[J].Soil Biology and Biochemistry,2010,42:2153-2160. |
| 41 | Doornbos R F,van Loon L C,Bakker P A H M.Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere:a review[J].Agronomy for Sustainable Development,2012,32:227-243. |
| 42 | Lugtenberg B,Kamilova F.Plant-growth-promoting Rhizobacteria[J].Annual Review of Microbiology,2009,63:541-556. |
| 43 | Bauld J,Brock T D.Ecological studies of Chloroflexis,a gliding photosynthetic bacterium[J].Archiv fur Mikrobiologie,1973,92:267. |
| 44 | Acosta-Martinez V,Dowd S E,Sun Y,et al.Pyrosequencing analysis for characterization of soil bacterial populations as affected by an integrated livestock-cotton production system[J].Applied and Environmental Microbiology,2010,45:13-25. |
| 45 | Xavier R.Soil properties are key determinants for the development of exudate gradients in a rhizosphere simulation model[J].Soil Biology and Biochemistry,2010,42(2):210-219. |
| 46 | Maron J L,Marler M,Klironomos J N,et al.Soil fungal pathogens and the relationship between plant diversity and productivity[J].Ecology Letters,2011,14:36-41. |
| 47 | Bais H P,Weir T L,Perry L G,et al.The role of root exudates in rhizosphere interactions with plants and other organisms[J].Annual Review of Plant Biology,2006,57:233-266. |
| 48 | Compant S,Clément C,Sessitsch A.Plant growth-promoting bacteria in the rhizo-and endosphere of plants:their role,colonization,mechanisms involved and prospects for utilization[J].Soil Biology and Biochemtry,2010,42:669-78. |
| 49 | 赵叶舟,王浩铭,汪自强.豆科植物和根瘤菌在生态环境中的地位和作用[J].农业资源与环境学报,2013,30(4):7-12. |
| 50 | 陈文新.中国豆科植物根瘤菌资源多样性与系统发育[J].中国农业大学学报,2004,9(2):6-7. |
| 51 | Perret X,Steahelin C,Broughton W J.Molecular basis of symbiotic promiscuity[J].Microbiology and Molecular Biology Reviews,2000,64:180-201. |
| 52 | Liu Z H,Cao Y M,Zhou Q W,et al.Acrylamide biodegradation ability and plant growth-promoting properties of Variovoraxboronicumulans CGMCC 4969[J].Biodegradation,2013,24(6):855-64. |
| 53 | White D C,Sutton S D,Ringelberg D B.The genus Sphingomonas:physiology and ecology[J].Current Opinion in Biotechnology,1996,7:301-306. |
| [1] | 高承兵, 常宗强. 降水量对连古城自然保护区荒漠植被凋落物分解和氮状态的影响[J]. 中国沙漠, 2021, 41(2): 145-152. |
| [2] | 郭新新, 左小安, 岳平, 李香云, 赵生龙, 吕朋, 胡亚. 内蒙古荒漠草原沙生针茅(Stipa glareosa)、碱韭(Allium polyrhizum)和骆驼蓬(Peganum harmala)叶形态性状对土壤水氮耦合的响应[J]. 中国沙漠, 2021, 41(1): 137-144. |
| [3] | 周晓兵, 张丙昌, 张元明. 生物土壤结皮固沙理论与实践[J]. 中国沙漠, 2021, 41(1): 164-173. |
| [4] | 雷军, 杨逍虎, 刘红梅, 赵玉红, 范菊萍, 郭彩霞. 黑河流域中游荒漠典型区域植被生物量及其影响因素[J]. 中国沙漠, 2021, 41(1): 203-208. |
| [5] | 何远政, 黄文达, 赵昕, 吕朋, 王怀海. 气候变化对植物多样性的影响研究综述[J]. 中国沙漠, 2021, 41(1): 59-66. |
| [6] | 武磊, 李常斌, 王刘明, 谢旭红, 张媛, 魏健美. 基于ESA-LUC和MODIS-NDVI的西北干旱荒漠-绿洲体系分类阈值及应用[J]. 中国沙漠, 2020, 40(6): 139-150. |
| [7] | 郝梦宇, 秦龙君, 毛鹏, 罗婕纯一, 赵文利, 邱国玉. 基于无人机可见光影像的荒漠植被分布格局研究方法[J]. 中国沙漠, 2020, 40(6): 169-179. |
| [8] | 王琨, 淮孟姣. 科学计量视角下的荒漠化研究热点[J]. 中国沙漠, 2020, 40(6): 201-211. |
| [9] | 王德金, 赵文智, 周宏. 河西走廊中部荒漠砾幂特征及其对土壤水分入渗的影响[J]. 中国沙漠, 2020, 40(6): 233-241. |
| [10] | 董雪, 李永华, 张正国, 李思瑶, 包岩峰, 郝玉光, 姚斌. 甘肃酒泉荒漠戈壁灌木群落优势物种生态位特征[J]. 中国沙漠, 2020, 40(4): 138-145. |
| [11] | 张润霞, 赵学勇, 李晶, 吕文强, 柴媛媛, 岳红琴. 干旱荒漠区土地利用方式快速转变对土壤入渗性能的影响[J]. 中国沙漠, 2020, 40(4): 146-153. |
| [12] | 陈应武, 陈庆霄, 杨昊天. 腾格里沙漠陆生野生脊椎动物多样性及区系[J]. 中国沙漠, 2020, 40(4): 171-182. |
| [13] | 张彩霞, 娄俊鹏, 蔡迪文. 荒漠地区15种植物的元素含量[J]. 中国沙漠, 2020, 40(4): 18-23. |
| [14] | 陈应武, 陈庆霄, 杨昊天. 腾格里沙漠昆虫多样性及区系特征[J]. 中国沙漠, 2020, 40(4): 216-222. |
| [15] | 刘淑娟, 魏兴琥, 郑倩倩, 林啟霞, 罗小兰, 陈毅哲, 梁钊雄, 关共凑. 西藏阿里高寒荒漠区土壤有机碳含量特征[J]. 中国沙漠, 2020, 40(4): 234-240. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
©2018中国沙漠 编辑部
地址: 兰州市天水中路8号 (730000)
电话:0931-8267545
Email:caiedit@lzb.ac.cn;desert@lzb.ac.cn
