中国沙漠 ›› 2020, Vol. 40 ›› Issue (5): 130-141.DOI: 10.7522/j.issn.1000-694X.2020.00015
周虹a,2( ), 吴波a,2(
), 吴波a,2( ), 高莹a,2, 成龙a, 贾晓红a,2, 庞营军a, 赵河聚a
), 高莹a,2, 成龙a, 贾晓红a,2, 庞营军a, 赵河聚a
收稿日期:2020-02-12
修回日期:2020-03-25
出版日期:2020-09-28
发布日期:2020-09-28
通讯作者:
吴波
作者简介:吴波(E-mail: wubo@caf.ac.cn)基金资助:
Hong Zhoua,2( ), Bo Wua,2(
), Bo Wua,2( ), Ying Gaoa,2, Long Chenga, Xiaohong Jiaa,2, Yingjun Panga, Heju Zhaoa
), Ying Gaoa,2, Long Chenga, Xiaohong Jiaa,2, Yingjun Panga, Heju Zhaoa
Received:2020-02-12
Revised:2020-03-25
Online:2020-09-28
Published:2020-09-28
Contact:
Bo Wu
摘要:
臭柏(Sabina vulgaris)是毛乌素沙地的主要固沙植物。臭柏群落中广泛分布的生物土壤结皮对维持沙地生态系统稳定具有重要意义。细菌是生物土壤结皮的重要组成部分,在维持生物土壤结皮结构和功能方面发挥着重要作用,但细菌群落组成及多样性随生物土壤结皮发育的变化尚不完全清楚。采用Illumina测序技术,分析了毛乌素沙地臭柏群落不同发育阶段生物土壤结皮(微生物结皮、藻结皮、地衣结皮和苔藓结皮)与裸沙的细菌群落组成及多样性,探究影响细菌群落结构的主要环境因子。结果表明:在毛乌素沙地随生物土壤结皮发育,细菌群落的多样性显著增加(P<0.05),苔藓结皮细菌群落多样性最高。生物土壤结皮的细菌群落以变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、蓝藻门(Cyanobacteria)和酸杆菌门(Acidobacteria)为优势菌群,这4个门的相对丰度均占各发育阶段生物土壤结皮细菌总丰度的78%以上。随生物土壤结皮发育,细菌群落组成发生显著变化,抗逆性较强的寡营养类群,如厚壁菌门(Firmicutes)和变形菌门(Proteobacteria)的相对丰度逐渐降低(P<0.05);富营养类群,如放线菌门(Actinobacteria)、酸杆菌门(Acidobacteria)、拟杆菌门(Bacteroidetes)和绿弯菌门(Chloroflexi)的相对丰度逐渐增加(P<0.05);蓝藻门(Cyanobacteria)在藻结皮阶段的相对丰度显著高于其他发育阶段(P<0.05)。群落组成的变化预示着生物土壤结皮细菌群落的生态功能由结皮发育初期通过促进土壤颗粒胶结来增加土壤表面的稳定性,转变为结皮发育后期通过固定碳氮和凋落物分解来促进生态系统的物质循环。细菌群落是生物土壤结皮发育过程中水分和养分变化的敏感指标,结皮层含水量、全碳、有机碳、全氮、硝态氮和全磷含量是促使生物土壤结皮细菌群落组成发生变化的主要环境因子。
中图分类号:
周虹, 吴波, 高莹, 成龙, 贾晓红, 庞营军, 赵河聚. 毛乌素沙地臭柏(Sabina vulgaris)群落生物土壤结皮细菌群落组成及其影响因素[J]. 中国沙漠, 2020, 40(5): 130-141.
Hong Zhou, Bo Wu, Ying Gao, Long Cheng, Xiaohong Jia, Yingjun Pang, Heju Zhao. Composition and influencing factors of the biological soil crust bacterial communities in the Sabina vulgaris community in Mu Us Sandy Land[J]. Journal of Desert Research, 2020, 40(5): 130-141.
| 项目 | 裸沙 | 微生物结皮 | 藻结皮 | 地衣结皮 | 苔藓结皮 |
|---|---|---|---|---|---|
| 土壤含水量/% | 5.27±0.11d | 12.67±1.50c | 15.11±0.90c | 18.66±1.78b | 22.25±1.49a |
| 有机碳/(g·kg-1) | 0.80±0.07d | 6.24±0.41c | 11.74±0.83b | 16.47±1.35b | 20.92±0.58a |
| 全碳/(g·kg-1) | 2.27±0.18d | 15.44±1.64c | 20.05±2.01c | 24.22±0.52b | 28.71±3.93a |
| 全氮/(g·kg-1) | 0.37±0.11c | 0.76±0.15b | 0.76±0.09b | 0.79±0.09b | 1.33±0.18a |
| 硝态氮/(mg·kg-1) | 1.61±0.03a | 1.60±0.02a | 1.32±0.07b | 0.87±0.07c | 0.68±0.10c |
| 铵态氮/(mg·kg-1) | 0.48±0.16 | 0.51±0.19 | 0.55±0.09 | 0.53±0.04 | 0.58±0.15 |
| 全磷/(g·kg-1) | 0.51±0.05b | 0.53±0.06b | 0.65±0.06a | 0.66±0.04a | 0.68±0.02a |
| pH | 7.07±0.03a | 7.01±0.01a | 7.02±0.01a | 6.91±0.03b | 6.89±0.01b |
表1 不同发育阶段结皮层理化性质(平均值±标准误差)
Table 1 Physicochemical characteristics in four developmental stages of biological soil crusts (mean±SE)
| 项目 | 裸沙 | 微生物结皮 | 藻结皮 | 地衣结皮 | 苔藓结皮 |
|---|---|---|---|---|---|
| 土壤含水量/% | 5.27±0.11d | 12.67±1.50c | 15.11±0.90c | 18.66±1.78b | 22.25±1.49a |
| 有机碳/(g·kg-1) | 0.80±0.07d | 6.24±0.41c | 11.74±0.83b | 16.47±1.35b | 20.92±0.58a |
| 全碳/(g·kg-1) | 2.27±0.18d | 15.44±1.64c | 20.05±2.01c | 24.22±0.52b | 28.71±3.93a |
| 全氮/(g·kg-1) | 0.37±0.11c | 0.76±0.15b | 0.76±0.09b | 0.79±0.09b | 1.33±0.18a |
| 硝态氮/(mg·kg-1) | 1.61±0.03a | 1.60±0.02a | 1.32±0.07b | 0.87±0.07c | 0.68±0.10c |
| 铵态氮/(mg·kg-1) | 0.48±0.16 | 0.51±0.19 | 0.55±0.09 | 0.53±0.04 | 0.58±0.15 |
| 全磷/(g·kg-1) | 0.51±0.05b | 0.53±0.06b | 0.65±0.06a | 0.66±0.04a | 0.68±0.02a |
| pH | 7.07±0.03a | 7.01±0.01a | 7.02±0.01a | 6.91±0.03b | 6.89±0.01b |
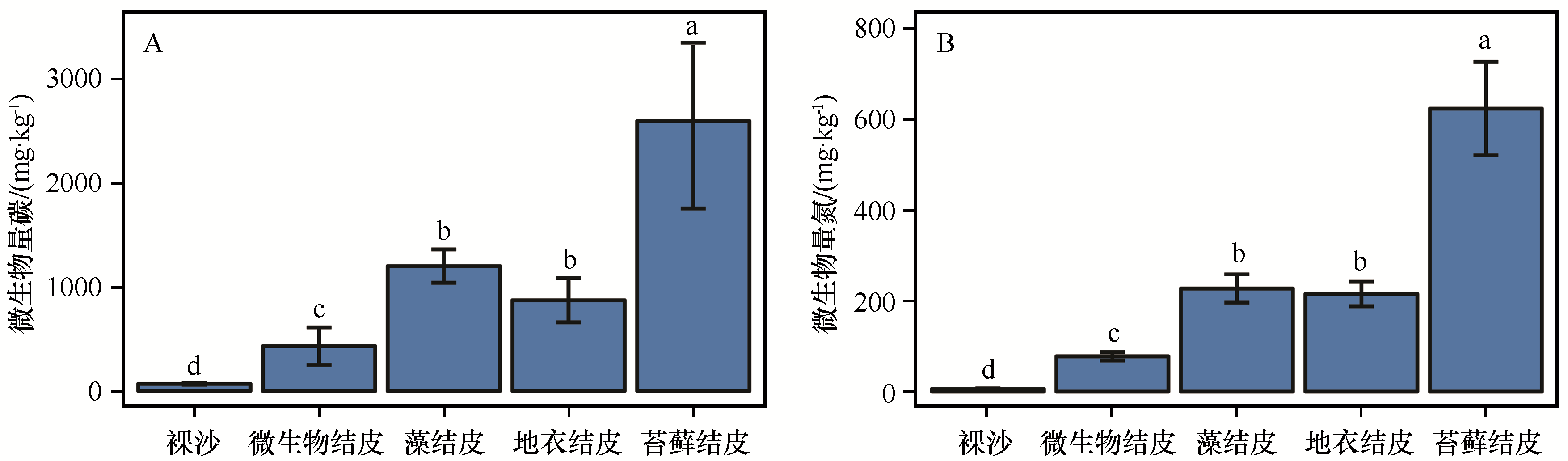
图1 不同发育阶段的生物土壤结皮微生物生物量碳和微生物生物量氮误差线表示标准误差(n=3),不同字母表示差异显著(P<0.05)
Fig.1 Microbial biomass carbon and microbial biomass nitrogen of different developmental stages of biological soil crusts
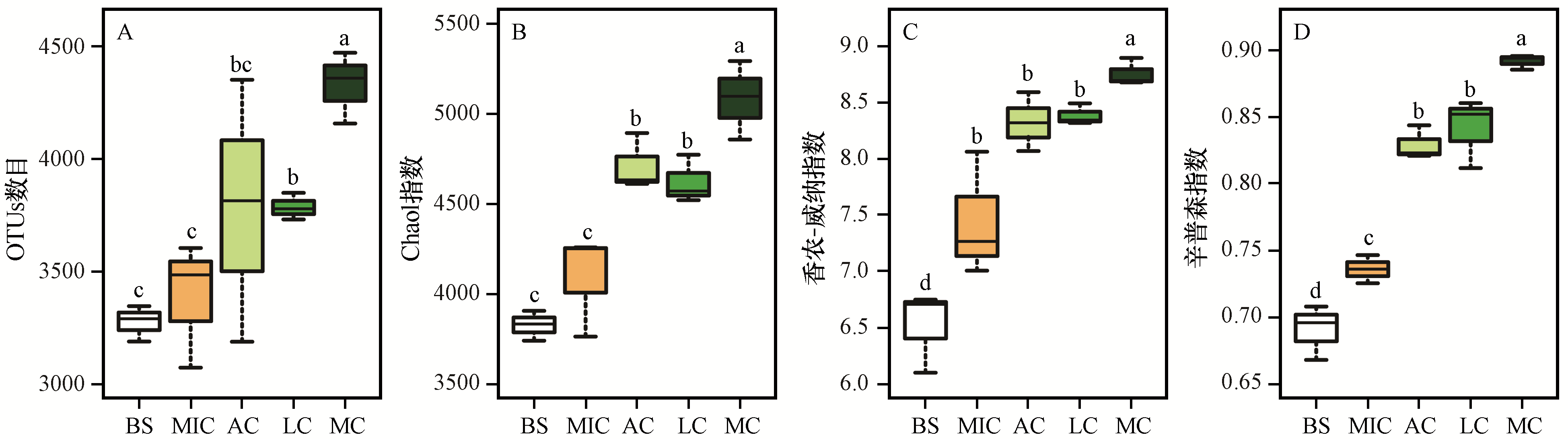
图2 不同发育阶段生物土壤结皮细菌多样性指数BS,裸沙;MIC,微生物结皮;AC,藻结皮;LC,地衣结皮;MC,苔藓结皮。不同字母表示差异显著(P<0.05)
Fig.2 Diversity index of the bacterial communities in different developmental stages of biological soil crusts
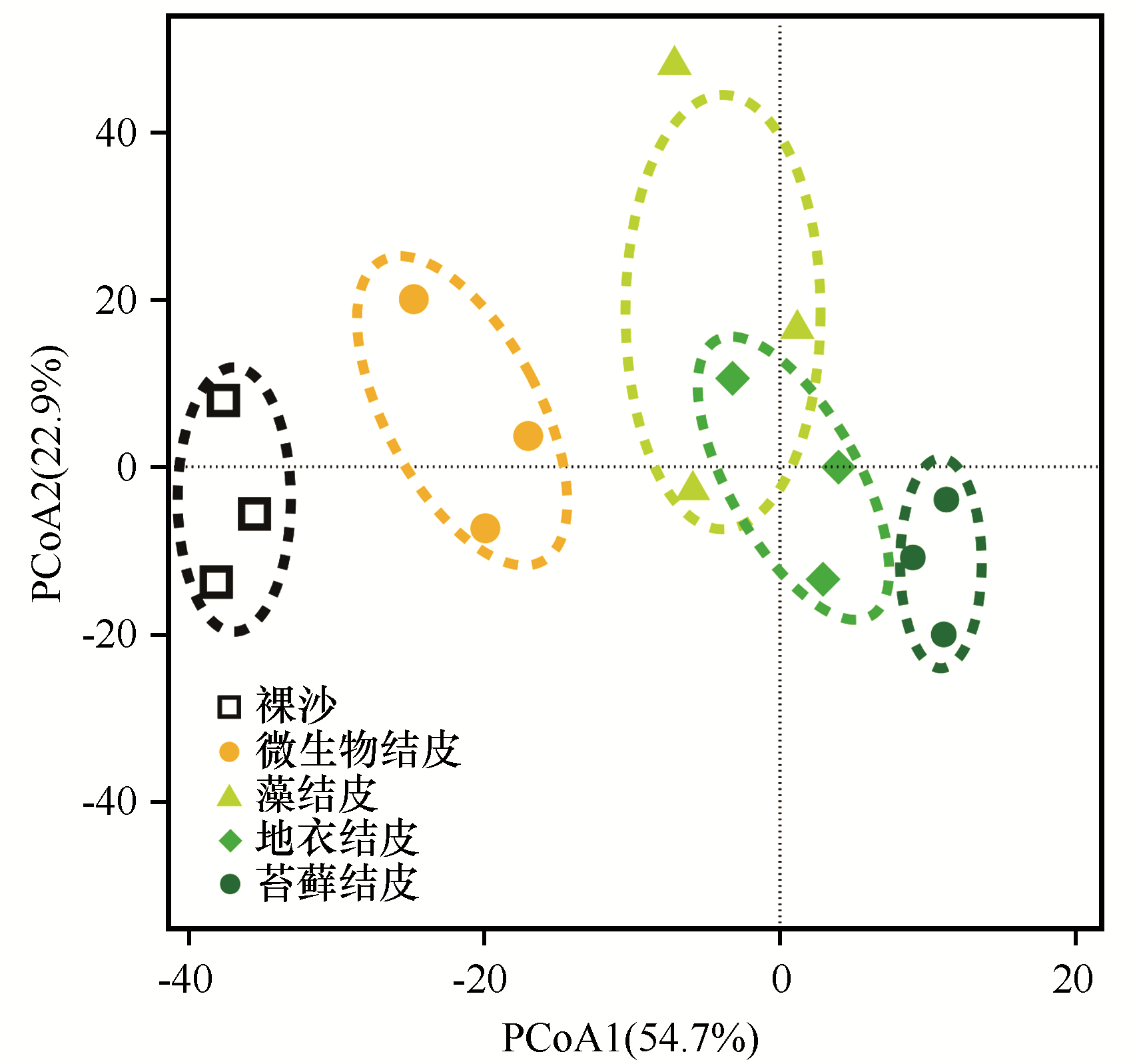
图3 不同发育阶段生物土壤结皮细菌群落主坐标分析(PcoA)
Fig. 3 Principal coordinates analysis (PCoA) of the bacterial communities in different developmental stages of biological soil crusts
| 样品分组 | ANOSIM | MRPP | Adonis | |||
|---|---|---|---|---|---|---|
| R | P | A | P | R2 | P | |
| 裸沙-微生物结皮 | 0.986 | 0.002 | 0.009 | 0.003 | 0.746 | 0.002 |
| 裸沙-藻结皮 | 0.926 | 0.014 | 0.087 | 0.021 | 0.594 | 0.018 |
| 裸沙-地衣结皮 | 0.996 | 0.008 | 0.051 | 0.004 | 0.684 | 0.006 |
| 裸沙-苔藓结皮 | 0.979 | 0.003 | 0.160 | 0.028 | 0.793 | 0.022 |
| 微生物结皮-藻结皮 | 0.370 | 0.048 | 0.013 | 0.041 | 0.184 | 0.039 |
| 微生物结皮-地衣结皮 | 0.926 | 0.009 | 0.106 | 0.039 | 0.364 | 0.028 |
| 微生物结皮-苔藓结皮 | 0.778 | 0.028 | 0.120 | 0.026 | 0.376 | 0.004 |
| 藻结皮-地衣结皮 | 0.222 | 0.045 | 0.012 | 0.023 | 0.210 | 0.047 |
| 藻结皮-苔藓结皮 | 0.370 | 0.039 | 0.022 | 0.035 | 0.195 | 0.036 |
| 地衣结皮-苔藓结皮 | 0.481 | 0.033 | 0.104 | 0.020 | 0.358 | 0.011 |
表2 不同发育阶段生物土壤结皮细菌群落组成的不相似检验
Table 2 Dissimilarity tests of the bacterial community compositions in different developmental stages of biological soil crusts
| 样品分组 | ANOSIM | MRPP | Adonis | |||
|---|---|---|---|---|---|---|
| R | P | A | P | R2 | P | |
| 裸沙-微生物结皮 | 0.986 | 0.002 | 0.009 | 0.003 | 0.746 | 0.002 |
| 裸沙-藻结皮 | 0.926 | 0.014 | 0.087 | 0.021 | 0.594 | 0.018 |
| 裸沙-地衣结皮 | 0.996 | 0.008 | 0.051 | 0.004 | 0.684 | 0.006 |
| 裸沙-苔藓结皮 | 0.979 | 0.003 | 0.160 | 0.028 | 0.793 | 0.022 |
| 微生物结皮-藻结皮 | 0.370 | 0.048 | 0.013 | 0.041 | 0.184 | 0.039 |
| 微生物结皮-地衣结皮 | 0.926 | 0.009 | 0.106 | 0.039 | 0.364 | 0.028 |
| 微生物结皮-苔藓结皮 | 0.778 | 0.028 | 0.120 | 0.026 | 0.376 | 0.004 |
| 藻结皮-地衣结皮 | 0.222 | 0.045 | 0.012 | 0.023 | 0.210 | 0.047 |
| 藻结皮-苔藓结皮 | 0.370 | 0.039 | 0.022 | 0.035 | 0.195 | 0.036 |
| 地衣结皮-苔藓结皮 | 0.481 | 0.033 | 0.104 | 0.020 | 0.358 | 0.011 |
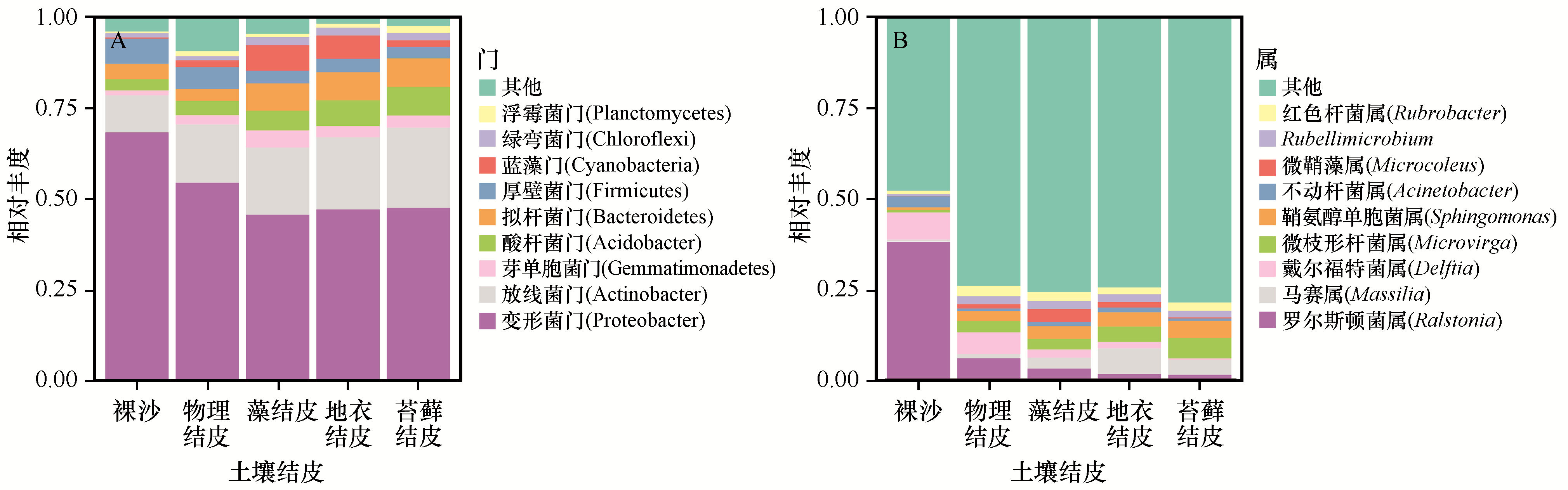
图4 不同发育阶段土壤结皮在门水平(A)和属水平(B)细菌群落组成
Fig.4 Bacterial community composition in different developmental stages of soil crusts at (A) phylum and (B) genus level
| 分类水平 | 物种 | 相对丰度/% | ||||
|---|---|---|---|---|---|---|
| 裸沙 | 微生物结皮 | 藻结皮 | 地衣结皮 | 苔藓结皮 | ||
| 门水平 | 放线菌 (Actinobacteria) | 10.3±0.5c | 16.8±2.2b | 19.0±0.3a | 19.9±1.5a | 22.3±1.5a |
| 酸杆菌 (Acidobacteria) | 3.1±0.3c | 4.2±0.8b | 5.6±0.7a | 7.1±0.4a | 8.0±0.3a | |
| 拟杆菌 (Bacteroidetes) | 3.3±0.9b | 3.4±1.0b | 7.7±1.4a | 7.8±0.5a | 7.9±0.4a | |
| 绿弯菌 (Chloroflexi) | 1.2±0.3b | 1.2±0.1b | 2.1±0.2a | 2.2±0.2a | 2.2±0.1a | |
| 蓝藻 (Cyanobacteria) | 0.3±0.1d | 2.0±0.8c | 7.2±0.7a | 6.4±0.4b | 1.8±0.6c | |
| 变形菌 (Proteobacteria) | 69.3±2.3a | 57.2±1.7a | 47.1±8.1b | 47.3±1.8b | 48.1±1.5b | |
| 厚壁菌 (Firmicutes) | 7.0±1.1a | 6.4±0.7a | 3.6±0.3b | 3.7±0.5b | 3.2±1.0c | |
| 芽单胞菌(Gemmatimonadetes) | 1.3±0.7a | 2.6±0.7a | 3.6±0.5a | 3.1±0.6a | 3.4±1.5a | |
| 浮霉菌 (Planctomycetes) | 0.5±0.2a | 1.5±0.5a | 0.9±0.5a | 1.1±0.3a | 1.6±0.2a | |
| 属水平 | 鞘氨醇单胞菌(Sphingomonas) | 0.7±0.3c | 3.0±0.3b | 3.6±1.3ab | 3.9±0.6a | 4.7±0.6a |
| 马赛菌 (Massilia) | 0.6±0.2d | 1.3±0.3c | 3.2±0.3b | 6.9±1.4a | 4.1±1.6a | |
| 微枝形杆菌 (Microvirga) | 0.7±0.2c | 3.4±0.6b | 3.1±0.5b | 4.1±0.5b | 5.7±0.3a | |
| 微鞘藻 (Microcoleus) | 0.1±0.1c | 1.3±0.4b | 3.8±1.0a | 1.5±0.2b | 0.3±0.1c | |
| 罗尔斯顿菌 (Ralstonia) | 38.0±5.5a | 6.6±0.4b | 3.3±0.5c | 1.6±0.8c | 1.5±0.1c | |
| 戴尔福特菌 (Delftia) | 7.4±0.8a | 6.5±0.4a | 2.4±0.3b | 1.7±0.4b | 0.4±0.1c | |
| 不动杆菌 (Acinetobacter) | 3.0±1.4a | 0.7±0.6b | 1.2±1.6ab | 1.3±0.1ab | 0.7±0.4b | |
| Rubellimicrobium | 0.6±0.5a | 2.4±1.1a | 2.4±1.4a | 2.1±0.6a | 1.8±0.7ab | |
| 红色杆菌 (Rubrobacter) | 0.9±0.3b | 3.1±0.5a | 2.6±1.0a | 1.8±0.8ab | 2.3±1.1a | |
表3 不同发育阶段生物土壤结皮细菌的相对丰度
Table 3 The relative abundance of bacteria (>1%) in different developmental stages of BSCs
| 分类水平 | 物种 | 相对丰度/% | ||||
|---|---|---|---|---|---|---|
| 裸沙 | 微生物结皮 | 藻结皮 | 地衣结皮 | 苔藓结皮 | ||
| 门水平 | 放线菌 (Actinobacteria) | 10.3±0.5c | 16.8±2.2b | 19.0±0.3a | 19.9±1.5a | 22.3±1.5a |
| 酸杆菌 (Acidobacteria) | 3.1±0.3c | 4.2±0.8b | 5.6±0.7a | 7.1±0.4a | 8.0±0.3a | |
| 拟杆菌 (Bacteroidetes) | 3.3±0.9b | 3.4±1.0b | 7.7±1.4a | 7.8±0.5a | 7.9±0.4a | |
| 绿弯菌 (Chloroflexi) | 1.2±0.3b | 1.2±0.1b | 2.1±0.2a | 2.2±0.2a | 2.2±0.1a | |
| 蓝藻 (Cyanobacteria) | 0.3±0.1d | 2.0±0.8c | 7.2±0.7a | 6.4±0.4b | 1.8±0.6c | |
| 变形菌 (Proteobacteria) | 69.3±2.3a | 57.2±1.7a | 47.1±8.1b | 47.3±1.8b | 48.1±1.5b | |
| 厚壁菌 (Firmicutes) | 7.0±1.1a | 6.4±0.7a | 3.6±0.3b | 3.7±0.5b | 3.2±1.0c | |
| 芽单胞菌(Gemmatimonadetes) | 1.3±0.7a | 2.6±0.7a | 3.6±0.5a | 3.1±0.6a | 3.4±1.5a | |
| 浮霉菌 (Planctomycetes) | 0.5±0.2a | 1.5±0.5a | 0.9±0.5a | 1.1±0.3a | 1.6±0.2a | |
| 属水平 | 鞘氨醇单胞菌(Sphingomonas) | 0.7±0.3c | 3.0±0.3b | 3.6±1.3ab | 3.9±0.6a | 4.7±0.6a |
| 马赛菌 (Massilia) | 0.6±0.2d | 1.3±0.3c | 3.2±0.3b | 6.9±1.4a | 4.1±1.6a | |
| 微枝形杆菌 (Microvirga) | 0.7±0.2c | 3.4±0.6b | 3.1±0.5b | 4.1±0.5b | 5.7±0.3a | |
| 微鞘藻 (Microcoleus) | 0.1±0.1c | 1.3±0.4b | 3.8±1.0a | 1.5±0.2b | 0.3±0.1c | |
| 罗尔斯顿菌 (Ralstonia) | 38.0±5.5a | 6.6±0.4b | 3.3±0.5c | 1.6±0.8c | 1.5±0.1c | |
| 戴尔福特菌 (Delftia) | 7.4±0.8a | 6.5±0.4a | 2.4±0.3b | 1.7±0.4b | 0.4±0.1c | |
| 不动杆菌 (Acinetobacter) | 3.0±1.4a | 0.7±0.6b | 1.2±1.6ab | 1.3±0.1ab | 0.7±0.4b | |
| Rubellimicrobium | 0.6±0.5a | 2.4±1.1a | 2.4±1.4a | 2.1±0.6a | 1.8±0.7ab | |
| 红色杆菌 (Rubrobacter) | 0.9±0.3b | 3.1±0.5a | 2.6±1.0a | 1.8±0.8ab | 2.3±1.1a | |
| 门 | 含水量 | 全碳 | 有机碳 | 全氮 | 硝态氮 | 铵态氮 | 全磷 | pH |
|---|---|---|---|---|---|---|---|---|
| 细菌群落 | 0.539** | 0.424* | 0.517* | 0.352* | 0.441* | 0.167 | 0.392* | 0.044 |
| 放线菌(Actinobacteria) | 0.521* | 0.329** | 0.550** | 0.525* | 0.332* | 0.045 | 0.368* | 0.394 |
| 酸杆菌(Acidobacteria) | 0.304 | 0.543* | 0.498* | 0.636* | 0.109* | 0.143 | 0.379 | 0.152* |
| 拟杆菌(Bacteroidetes) | 0.646** | 0.536* | 0.800* | 0.304 | 0.115* | 0.177 | 0.386 | -0.211 |
| 绿弯菌(Chloroflexi) | 0.296* | 0.436 | 0.369* | 0.500 | 0.209 | 0.246 | 0.761* | 0.143 |
| 蓝藻(Cyanobacteria) | 0.246 | 0.268 | 0.742 | 0.332 | 0.235* | 0.696* | 0.696 | 0.199 |
| 变形菌(Proteobacteria) | -0.246* | -0.443 | -0.634 | -0.593** | 0.321* | -0.234 | -0.864* | -0.322 |
| 厚壁菌(Firmicutes) | -0.654* | -0.150 | -0.391 | -0.336 | 0.177 | -0.028 | -0.293 | -0.252 |
| 芽单胞菌(Gemmatimonadetes) | 0.271 | 0.239 | 0.284 | 0.429 | 0.273 | 0.314 | 0.511 | 0.442 |
| 浮霉菌(Planctomycetes) | 0.364 | 0.486 | 0.336 | 0.686* | 0.353 | 0.102 | 0.868 | 0.032 |
表4 生物土壤结皮细菌群落与土壤环境因子的相关性
Table 4 Correlations between soil environmental factors and bacterial communities in biological soil crusts
| 门 | 含水量 | 全碳 | 有机碳 | 全氮 | 硝态氮 | 铵态氮 | 全磷 | pH |
|---|---|---|---|---|---|---|---|---|
| 细菌群落 | 0.539** | 0.424* | 0.517* | 0.352* | 0.441* | 0.167 | 0.392* | 0.044 |
| 放线菌(Actinobacteria) | 0.521* | 0.329** | 0.550** | 0.525* | 0.332* | 0.045 | 0.368* | 0.394 |
| 酸杆菌(Acidobacteria) | 0.304 | 0.543* | 0.498* | 0.636* | 0.109* | 0.143 | 0.379 | 0.152* |
| 拟杆菌(Bacteroidetes) | 0.646** | 0.536* | 0.800* | 0.304 | 0.115* | 0.177 | 0.386 | -0.211 |
| 绿弯菌(Chloroflexi) | 0.296* | 0.436 | 0.369* | 0.500 | 0.209 | 0.246 | 0.761* | 0.143 |
| 蓝藻(Cyanobacteria) | 0.246 | 0.268 | 0.742 | 0.332 | 0.235* | 0.696* | 0.696 | 0.199 |
| 变形菌(Proteobacteria) | -0.246* | -0.443 | -0.634 | -0.593** | 0.321* | -0.234 | -0.864* | -0.322 |
| 厚壁菌(Firmicutes) | -0.654* | -0.150 | -0.391 | -0.336 | 0.177 | -0.028 | -0.293 | -0.252 |
| 芽单胞菌(Gemmatimonadetes) | 0.271 | 0.239 | 0.284 | 0.429 | 0.273 | 0.314 | 0.511 | 0.442 |
| 浮霉菌(Planctomycetes) | 0.364 | 0.486 | 0.336 | 0.686* | 0.353 | 0.102 | 0.868 | 0.032 |
| 1 | 李新荣,张景光,王新平,等.干旱沙漠区土壤微生物结皮及其对固沙植被影响的研究[J].植物学报,2000,42(9):965-970. |
| 2 | Liu L,Liu Y,Peng Z,et al.Development of bacterial communities in biological soil crusts along a revegetation chronosequence in the Tengger Desert, northwest China[J].Biogeosciences,2017,14(16):1-25. |
| 3 | Belnap J.The world at your feet: desert biological soil crusts[J].Frontiers in Ecology & the Environment,2003,1(4):181-189. |
| 4 | 李新荣,谭会娟,回嵘,等.中国荒漠与沙地生物土壤结皮研究[J].科学通报,2018,63(23):16-30. |
| 5 | Faist A M,Herrick J E,Belnap J,et al.Biological soil crust and disturbance controls on surface hydrology in a semi-arid ecosystem[J].Ecosphere,2017,8(3):e01691. |
| 6 | Adessi A,Carvalho R C D,Philippis R D,et al.Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts[J].Soil Biology & Biochemistry,2018,116:67-69. |
| 7 | 都军,李宜轩,杨晓霞,等.腾格里沙漠东南缘生物土壤结皮对土壤理化性质的影响[J].中国沙漠,2018(1):111-116. |
| 8 | 肖巍强,董志宝,陈颢,等.生物土壤结皮对库布齐沙漠北缘土壤粒度特征的影响[J].中国沙漠,2017(5):970-977. |
| 9 | Jorge-Villar S,Edwards H.Microorganism response to stressed terrestrial environments: a raman spectroscopic perspective of extremophilic life strategies[J].Life Open Access Journal,2013,3(1):276-294. |
| 10 | Mager D M,Thomas A D.Extracellular polysaccharides from cyanobacterial soil crusts: a review of their role in dryland soil processes[J].Journal of Arid Environments,2011,75(2):91-97. |
| 11 | Muñoz-Rojas M,Román J R,Roncero-Ramos B, et al.Cyanobacteria inoculation enhances carbon sequestration in soil substrates used in dryland restoration[J].Science of the Total Environment,2018,636:1149-1154. |
| 12 | Elliott D R,Thomas A D,Hoon S R,et al.Niche partitioning of bacterial communities in biological crusts and soils under grasses, shrubs and trees in the Kalahari[J].Biodiversity & Conservation,2014,23(7):1709-1733. |
| 13 | Yu J,Glazer N,Steinberger Y.Carbon utilization, microbial biomass, and respiration in biological soil crusts in the Negev Desert[J].Biology & Fertility of Soils,2014,50(2):285-293. |
| 14 | Liu L C,Liu Y B,Hui R,et al.Recovery of microbial community structure of biological soil crusts in successional stages of Shapotou desert revegetation, northwest China[J].Soil Biology & Biochemistry,2017,107:125-128. |
| 15 | Zhang B C,Zhou X B,Zhang Y M.Responses of microbial activities and soil physical-chemical properties to the successional process of biological soil crusts in the Gurbantunggut Desert, Xinjiang[J].Journal of Arid Land,2015,7(1):101-109. |
| 16 | Nagy M L,Alejandro P,Ferran G P.The prokaryotic diversity of biological soil crusts in the Sonoran Desert (Organ Pipe Cactus National Monument, AZ)[J].Fems Microbiology Ecology,2010,54(2):233-245. |
| 17 | Abed R M M,Kharusi S A,Schramm A,et al.Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman[J].Fems Microbiology Ecology,2010,72(3):418-428. |
| 18 | Zhang B C,Kong W D,Wu N,et al.Bacterial diversity and community along the succession of biological soil crusts in the Gurbantunggut Desert, Northern China[J].Journal of Basic Microbiology,2016,56(6):670-679. |
| 19 | Maier S,Schmidt T S B,Zheng L,et al.Analyses of dryland biological soil crusts highlight lichens as an important regulator of microbial communities[J].Biodiversity & Conservation,2014,23(7):1735-1755. |
| 20 | Gundlapally S R,Garciapichel F.The community and phylogenetic diversity of biological soil crusts in the colorado plateau studied by molecular fingerprinting and intensive cultivation[J].Microbial Ecology,2006,52(2):345-357. |
| 21 | Redfield E,Barns S M,Belnap J,et al.Comparative diversity and composition of cyanobacteria in three predominant soil crusts of the Colorado Plateau [J].Fems Microbiology Ecology,2006,40(1):55-63. |
| 22 | Zhang Q Y,Wang Q,Ouyang H L,et al.Pyrosequencing reveals significant changes in microbial communities along the ecological succession of biological soil crusts in the Tengger desert of China[J].Pedosphere,2018,28(2):186-198. |
| 23 | 杨丽娜,赵允格,明姣,等.黄土高原不同侵蚀类型区生物结皮中蓝藻的多样性[J].生态学报,2013,33(14):4416-4424. |
| 24 | Hagemann M,Henneberg M,Felde V J M N L,et al.Cyanobacterial populations in biological soil crusts of the northwest Negev Desert, Israel-effects of local conditions and disturbance[J].Fems Microbiology Ecology,2017,93(6):fiw228. |
| 25 | Zhang R F,Cui Z,Li S.Advance in methods for research on soil microbial community structure[J].Soils,2004,36(5):476-437. |
| 26 | Mueller R C,Belnap J,Kuske C R.Soil bacterial and fungal community responses to nitrogen addition across soil depth and microhabitat in an arid shrubland[J].Frontiers in Microbiology,2015,6:891. |
| 27 | 吴波.毛乌素沙地的景观动态与荒漠化成因研究[D].北京:中国科学院地理科学与资源研究所,1997. |
| 28 | 陈昌笃.走向宏观生态学:陈昌笃论文集[M].北京:科学出版社,2009. |
| 29 | 张军红,吴波.油蒿与臭柏沙地生物结皮对土壤理化性质的影响[J].东北林业大学学报,2012,40(3):58-61. |
| 30 | 郭爱莲,张卫兵,朱志诚,等.固沙植物臭柏的死亡原因及保护对策[J].水土保持通报,2002,22(2):16-18. |
| 31 | 北京大学地理系,中国科学院自然资源综合考察委员会,中国科学院兰州沙漠研究所,等.毛乌素沙区自然条件及其改良利用[M].北京:科学出版社,1983. |
| 32 | Wu B,Ci L J.Landscape change and desertification development in the Mu Us Sandland, Northern China[J].Journal of Arid Environments,2002,50(3):429-444. |
| 33 | 王林和,党宏忠,张国盛,等.中国天然臭柏群落的分布与生物量特征[J].内蒙古农业大学学报(自然科学版),2014(1):37-45. |
| 34 | Lawley B,Tannock G W.Analysis of 16S rRNA gene amplicon sequences using the QIIME software package[J].Methods in Molecular Biology,2017,1537:153. |
| 35 | 杨航宇,刘艳梅,王廷璞,等.生物土壤结皮对荒漠区土壤微生物数量和活性的影响[J].中国沙漠,2017,37(5):950-960. |
| 36 | Zhang B,Zhou X,Zhang Y.Responses of microbial activities and soil physical-chemical properties to the successional process of biological soil crusts in the Gurbantunggut Desert, Xinjiang[J].Journal of Arid Land,2015,7. |
| 37 | Xu X,Thornton P E,Post W M.A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems[J].Global Ecology & Biogeography,2013,22(6):737-749. |
| 38 | Mogul R,Vaishampayan P,Bashir M,et al.Microbial community and biochemical dynamics of biological soil crusts across a gradient of surface coverage in the central Mojave Desert[J].Frontiers in Microbiology,2017,8:1974. |
| 39 | 李靖宇,张琇.腾格里沙漠不同生物土壤结皮微生物多样性分析[J].生态科学,2017,36(3):36-42. |
| 40 | Cameron W,Klaus S,Samiran B,et al.Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning[J].Nature Communications,2019,10:4841. |
| 41 | Zhang B C,Kong W,Wu N,et al.Bacterial diversity and community along the succession of biological soil crusts in the Gurbantunggut Desert, Northern China[J].Journal of Basic Microbiology,2016,56(6):670-679. |
| 42 | Blaire S,Kuske C R,La Verne G G,et al.Climate change and physical disturbance manipulations result in distinct biological soil crust communities[J].Applied & Environmental Microbiology,2015,81(21):7448-7459. |
| 43 | Kuske C R,Yeager C M,Johnson S,et al.Response and resilience of soil biocrust bacterial communities to chronic physical disturbance in arid shrublands[J].Isme Journal,2012,6(4):886-897. |
| 44 | Mandic-Mulec I,Stefanic P,van Elsas J D.Ecology of bacillaceae[J].Microbiol Spectrum,2015,3(2):TBS-0017-2013. |
| 45 | Maier S,Tamm A,Wu D,et al.Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts[J].Isme Journal,2018,12(4):1032-1046. |
| 46 | Pepe-Ranney C,Koechli C,Potrafka R,et al.Non-cyanobacterial diazotrophs mediate dinitrogen fixation in biological soil crusts during early crust formation[J].Isme Journal,2016,10(2):287-298. |
| 47 | Maier S,Muggia L,Kuske C R,et al. Bacteria and non-lichenized fungi within biological soil crusts Biological soil crusts : an organizing principle in drylands[M]. Berlin, Germany:. Springer, 2016:81-100. |
| 48 | Lauber C L,Hamady M,Knight R,et al.Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale[J].Applied & Environmental Microbiology,2009,75(15):5111. |
| 49 | Makhalanyane T P,Angel V,Eoin G,et al.Microbial ecology of hot desert edaphic systems[J].Fems Microbiology Reviews,2015,39(2):203-221. |
| 50 | Lee O O,Wang Y,Yang J,et al.Pyrosequencing reveals highly diverse and species-specific microbial communities in sponges from the Red Sea[J].Isme Journal,2011,5(4):650. |
| 51 | Belnap J,Lange O L.Biological Soil Crusts:Structure, Function, and Management[M].Berlin,Germany:Springer-Verlag,2002. |
| 52 | Couradeau E,Giraldo-Silva A,Martini F D,et al.Spatial segregation of the biological soil crust microbiome around its foundational cyanobacterium, Microcoleus vaginatus , and the formation of a nitrogen-fixing cyanosphere[J].Microbiome,2019,7(1):55. |
| 53 | Yeager C M,Kornosky J L,Morgan R E,et al.Three distinct clades of cultured heterocystous cyanobacteria constitute the dominant N2-fixing members of biological soil crusts of the Colorado Plateau, USA[J].Fems Microbiology Ecology,2007,60(1):85-97. |
| 54 | Lan S,Li W,Zhang D,et al.Effects of drought and salt stresses on man-made cyanobacterial crusts[J].European Journal of Soil Biology,2010,46(6):381-386. |
| 55 | Pasternak Z,al ashhab A,Gatica J,et al.Spatial and temporal biogeography of soil microbial communities in arid and semiarid regions[J].PloS One,2013,8:e69705. |
| 56 | Dumbrell A J,Nelson M,Helgason T,et al.Relative roles of niche and neutral processes in structuring a soil microbial community[J].Isme Journal,2010,4(3):337-345. |
| 57 | Yao M,Rui J,Li J,et al.Rate-specific responses of prokaryotic diversity and structure to nitrogen deposition in the Leymus chinensis steppe[J].Soil Biology and Biochemistry,2014,79:81-90. |
| [1] | 周晓兵, 张丙昌, 张元明. 生物土壤结皮固沙理论与实践[J]. 中国沙漠, 2021, 41(1): 164-173. |
| [2] | 赵洋, 潘颜霞, 苏洁琼, 张志山. 中国干旱区沙化土地绿色环保治理技术综述[J]. 中国沙漠, 2021, 41(1): 195-202. |
| [3] | 王姣月, 秦树高, 张宇清. 毛乌素沙地植被水分利用效率的时空格局[J]. 中国沙漠, 2020, 40(5): 120-129. |
| [4] | 李夙超, 邱曼, 李孝泽, 刘景德, 王立民, 胡杨, 李志刚, 牛改红. 准噶尔盆地西缘白杨河上游莫合台冲积洪积扇戈壁的特征、时代及过程[J]. 中国沙漠, 2020, 40(4): 1-9. |
| [5] | 许明静, 吕萍, 肖南, 杨军怀, 刘铮瑶, 冯淼彦, 梁准. 毛乌素沙地西北部植被覆盖对沙丘移动的影响[J]. 中国沙漠, 2020, 40(4): 71-80. |
| [6] | 周立峰, 杨荣, 赵文智. 荒漠人工固沙植被区土壤结皮斥水性发展特征[J]. 中国沙漠, 2020, 40(3): 185-192. |
| [7] | 王岩松, 刘玉冰, 王增如, 赵丽娜, 漆婧华, 张雯莉. 生物土壤结皮铁代谢微生物组成及其功能基因对演替的响应[J]. 中国沙漠, 2020, 40(3): 193-200. |
| [8] | 赵雅姣, 刘晓静, 吴勇, 童长春. 豆禾牧草间作根际土壤养分、酶活性及微生物群落特征[J]. 中国沙漠, 2020, 40(3): 219-228. |
| [9] | 李想, 苏志珠, 马义娟, 张彩霞, 柳苗苗. 毛乌素沙地东南缘全新世气候不稳定性[J]. 中国沙漠, 2020, 40(2): 109-117. |
| [10] | 徐丹蕾, 丁靖南, 伍永秋. 1989-2014年毛乌素沙地湖泊面积[J]. 中国沙漠, 2019, 39(6): 40-47. |
| [11] | 张瑞, 周晓兵, 张元明. 生物土壤结皮对温带荒漠植物凋落物分解的影响[J]. 中国沙漠, 2019, 39(6): 151-158. |
| [12] | 刘艳梅, 杨航宇, 贾荣亮, 李宜轩. 人为踩踏生物土壤结皮对土壤酶活性的影响[J]. 中国沙漠, 2019, 39(4): 54-63. |
| [13] | 杨航宇, 刘长仲, 刘艳梅, 杨昊天. 荒漠区踩踏生物土壤结皮对土壤微生物量的影响[J]. 中国沙漠, 2019, 39(2): 35-44. |
| [14] | 韩瑞, 苏志珠, 李想, 柳苗苗, 马义娟. 粒度和磁化率记录的毛乌素沙地东缘全新世气候变化[J]. 中国沙漠, 2019, 39(2): 105-114. |
| [15] | 白壮壮, 崔建新. 近2 000 a毛乌素沙地沙漠化及成因[J]. 中国沙漠, 2019, 39(2): 177-185. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
©2018中国沙漠 编辑部
地址: 兰州市天水中路8号 (730000)
电话:0931-8267545
Email:caiedit@lzb.ac.cn;desert@lzb.ac.cn
